Summary
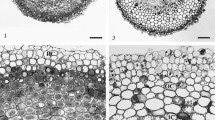
Mineral distribution in the roots of wheat (Triticum aestivum L. cv. Wheaton) was investigated using X-ray microanalysis of bulk frozen hydrated roots in SEM and of freeze substituted sections in TEM. Results obtained using the two methods agreed reasonably well. A total often elements were detected: Na, Mg, Si, P, S, Cl, K, Ca, Mn, and Fe. Of these Si, P, Ca, and Mn were incorporated into biomineralized structures. Silica was deposited in the endodermal walls in the older parts of the root. Silicon was also detected in the large central metaxylem lumina in the basal zone of the root, and in the smaller peripheral metaxylem and the immediately contiguous pericycle and outer parenchyma cells bridging the small metaxylem vessels to the endodermal layer. In the basal zone of the root some of the inner cortical cells contained intracellular electron opaque deposits. These were associated with the cell walls, had non-opaque inclusions and microanalysis revealed that they consisted of calcium, phosphorus and manganese.
Similar content being viewed by others
Abbreviations
- A:
-
apical zone of root
- M:
-
midzone of root
- B:
-
basal zone of root
- SEM:
-
scanning electron microscope
- TEM:
-
transmission electron microscope
References
Arnott HJ (1982) Three systems of biomineralization in plants with comments on the associated organic matrix. In: Nancollas GH (ed) Biological mineralization and demineralization. Dahlem Konferenzen 1982. Springer, Berlin Heidelberg New York, pp 199–218
Bennett DM (1982) Silicon deposition in the roots ofHordeum sativum Jess.,Avena sativa L. andTriticum aestivum L. Ann Bot 50: 239–245
DeMason DA, Stillman JI (1986) Identification of phosphate granules occurring in seedling tissue of two palm species (Phoenix dactylifera andWashingtonia filifera). Planta 167: 321–329
Gartner S, Le Faucheur L, Roinel N, Paris-Pireyre N (1984) Preliminary studies on the elemental composition from xylem exudate form two varieties of wheat by electron probe analysis. Scann Electron Microsc 1984: 1739–1744
Griffith M, Defoliart L (1988) Nitrogen, phosphate and starch storage inEriophorum vaginatum, an Arctic sedge. Poster paper presented at 21st plant development workshop, May 1988, University of Waterloo, Ontario, Canada
Harvey DMR (1985) The effects of salinity on ion concentrations within the root cells ofZea mays L. Planta 165: 242–248
—, Brandtner R (1987) The regulation of ion levels withinPlantago maritima roots: an examination of freeze substituted and frozen hydrated specimens. Acta Histochem Cytochem 20: 437–445
—, Stelzer R, Brandtner R, Kramer D (1986) Effects of salinity on ultrastructure and ion distributions in roots ofPlantago coronopus. Physiol Plant 66: 328–338
Hajibagheri MA, Harvey DMR, Flowers TJ (1987) Quantitative ion distribution within root cells of salt-sensitive and salt-tolerant maize varieties. New Phytol 105: 367–379
Harrison-Murray RS, Clarkson DT (1973) Relationships between structural development and absorption of ions by the root system ofCucurbita pepo. Planta 114: 1–16
Hodson MJ (1986) Silicon deposition in the roots, culm and leaf ofPhalaris canariensis L. Ann Bot 58: 167–177
—, Bell A (1986) The mineral relations of the lemma ofPhalaris canariensis L., with particular reference to its silicified macrohairs. Israel J Bot 35: 241–253
—, Sangster AG (1988) Observations on the distribution of mineral elements in the leaf of wheat (Triticum aestivum L.) with particular reference to silicon. Ann Bot 62: 463–471
Jarvis SC (1987) The uptake and transport of silicon by perennial ryegrass and wheat. Plant Soil 97: 429–438
Jeffrey DW (1968) Phosphate nutrition of Australian heath plants. II. The formation of polyphosphate by five heath species. Aust J Bot 16: 603–613
Kaufman PB, Dayanandan P, Takeoka Y, Bigelow WC, Jones JD, Iler R (1981) Silica in shoots of higher plants. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York Heidelberg Berlin, pp 409–449
Kramer D, Anderson WP, Preston J (1978) Transfer cells in the root epidermis ofAtriplex hastata L. as a response to salinity: a comparative cytological and X-ray microprobe investigation. Aust J Plant Physiol 5: 737–747
Lowenstam HA (1981) Minerals formed by organisms. Science 211: 1126–1131
McCully ME, Canny MJ, Van Steveninck, RFM (1987) Accumulation of potassium by differentiating metaxylem elements of maize roots. Physiol Plant 69: 73–80
Mann S (1988) Molecular recognition in biomineralization. Nature 332: 119–124
Markhart AH, Läuchli A (1982) The comparison of three freezing methods for electron probe X-ray microanalysis of hydrated barley tissue. Plant Sci Lett 25: 29–36
Metcalfe CR (1960) Anatomy of the monocotyledons. 1.Gramineae Oxford: Clarendon Press.
Parry DW, Hodson MJ, Sangster AG (1984) Some recent advances in studies of silicon in higher plants. Philos Trans R Soc Lond [Biol] 304: 537–549
Peggs A, Bowen H (1984) Inability to detect organosilicon compounds inEquisetum andThuja. Phytochemistry 23: 1788–1799
Perry CC, Williams RJP, Fry SC (1987) Cell wall biosynthesis during silicification of grass hairs. J Plant Physiol 126: 437–448
Pitman MG, Läuchli A, Stelzer R (1981) Ion distribution in roots of barley seedlings measured by electron probe X-ray microanalysis. Plant Physiol 68: 673–679
Reynolds ES (1963) The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J Cell Biol 17: 208–212
Sangster AG (1970 a) Intracellular silica deposition in immature leaves in three species ofGramineae. Ann Bot 34: 245–57
— (1970 b) Intracellular silica deposition in mature and senescent leaves ofSieglingia decumbens (L.) Bernh. Ann Bot 34: 557–570
—, Hodson MJ (1986) Silica in higher plants. Ciba Found Symp 121: 90–111
—, Parry DW (1976) The ultrastructure and electron-probe microassay of silicon deposits in the endodermis of the seminal roots ofSorghum bicolor (L.). Moench. Ann Bot 40: 447–459
— — (1981) Ultrastructure of silica deposits in higher plants. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York Heidelberg Berlin, pp 383–407
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Storey R, Walker RR (1987) Some effects of root anatomy on K, Na and Cl loading of citrus roots and leaves. J Exp Bot 38: 1769–1780
—, Pitman MG, Stelzer R (1983) X-ray micro-analysis of cells and cell compartments ofAtriplex spongiosa. J Exp Bot 34: 1196–1206
Weiss A, Herzog A (1978) Isolation and characterization of a siliconorganic complex from plants. In: Bendz G, Lindqvist I (eds) Biochemistry of silicon and related problems. Plenum, New York, pp 109–127
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hodson, M.J., Sangster, A.G. Subcellular localization of mineral deposits in the roots of wheat (Triticum aestivum L.). Protoplasma 151, 19–32 (1989). https://doi.org/10.1007/BF01403298
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01403298