Summary
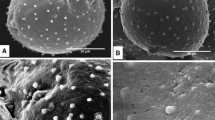
Numerous polysaccharide-rich particles (“P-particles”) occur in the tip region of growing grass pollen tubes, where they apparently contribute to the extending wall. In other families the corresponding bodies have been shown to originate from dictyosome activity during pollen tube growth. However, in the grasses the main synthesis precedes anthesis; the P-particles represent up to 30% of the reserves of the vegetative cell of the dormant grain, numbering over one million in the pollen grain of rye. Their membranes are incomplete. The polysaccharide content, which is initially coarsely granular but becomes microfibrillar with hydration, is readily extracted with ammonium oxalate, and is probably pectic in nature. Simple methods for isolating the particles in relatively pure populations are described. Hydrolysis yields principally galactose, arabinose, glucose, and rhamnose. Apart from proteins derived from the original bounding membranes, a protein fraction is tenaciously bound to the polysaccharide. Isolated P-particles move anodically in an electrical field, and the possibility that their movement from the grain to the tube tip during growth depends on a potential gradient, already demonstrated for lily pollen tubes, is considered.
Similar content being viewed by others
References
Albersheim, P., Mühlethaler, K., Frey-Wyssling, A., 1960: Stained pectin as seen in the electron microscope. J. biophys. biochem. Cytol.8, 501–506.
Blumenkrantz, N., Asboe-Hansen, G., 1973: New method for quantitative determination of uronic acids. Anal. Biochem.54, 484–489.
Bonnett, H. T., Newcomb, E. H., 1966: Coated vesicles and other cytoplasmic components of growing root hairs of radish. Protoplasma62, 59–75.
Cass, D. D., Peteya, D. J., 1979: Growth of barley pollen tubesin vivo. I. Ultrastructural aspects of early pollen tube growth in the stigmatic hair. Can. J. Bot.57, 386–396.
Colvin, J. R., Leppard, G. G., 1973: Fibrillar, modified polygalacturonic acid in, on and between plant cell walls. In: Biogenesis ofPlant Cell Wall Polysaccharides (Loewus, F., ed.), p. 315. New York-London: Academic Press.
Cresti, M., van Went, J. L., 1976: Callose deposition and plug formation inPetunia pollen tubesin situ. Planta133, 35–40.
Dashek, W. V., Rosen, W. G., 1966: Electron-microscopical localisation of chemical components in the growth zone ofLilium pollen tubes. Protoplasma61, 192–204.
De Nettancourt, D., Devreux, M., Bozzini, A., Cresti, M., Pacini, E., Sarfatti, G., 1973: Ultrastructural aspects of self-incompatibility mechanism inLycopersicum peruvianum Mill. J. Cell Sci.12, 403–419.
Dickinson, H. G., Lawson, J., 1975: Pollen tube growth in the stigma ofOenothera organensis following compatible and incompatible intraspecific pollinations. Proc. Roy. Soc.B188, 327–344.
— —, 1976: The growth of the pollen tube wall inOenothera organensis. J. Cell Sci.18, 519–525.
Engels, F. M., 1973: Function of Golgi vesicles in relation to cell wall synthesis in germinatingPetunia pollen. I. Isolation of Golgi vesicles. Acta Bot. Neerl.22, 6–13.
—, 1974 a: Function of Golgi vesicles in relation to cell wall synthesis in germinatingPetunia pollen. II. Chemical composition of Golgi vesicles and pollen tube wall. Acta Bot. Neerl.23, 81–89.
—, 1974 b: Function of Golgi vesicles in relation to cell wall synthesis in germinatingPetunia pollen. III. The ultrastructure of the tube wall. Acta Bot. Neerl.23, 201–208.
—, 1974 c: Function of Golgi vesicles in relation to cell wall synthesis in germinatingPetunia pollen. IV. Identification of cellulose in pollen tube walls and Golgi vesicles by X-ray diffraction. Acta Bot. Neerl.23, 209–216.
Grove, S. N., Bracker, C. E., Morré, D. J., 1970: An ultrastructural basis for hyphal tip growth inPythium ultimum. Amer. J. Bot.57, 245–266.
Heslop-Harrison, J., 1979 a: Aspects of the structure, cytochemistry and germination of the pollen of rye (Secale cereale L.). Ann. Bot. Suppl. No. 1, Vol.44, pp. 1–47.
—, 1979 b: Pollen-stigma interaction in the grasses: a brief review. N. Z. J. Bot.17, 537–546.
Heslop-Harrison, Y., Heslop-Harrison, J., 1979: The digestive glands ofPinguicula: fine-structure and cytochemistry. Ann. Bot.47, 293–319.
Jarvis, M. C., Hall, M. A., Threlfall, D. R., Friend, J., 1981: The polysaccharide structure of potato cell walls: chemical fractionation. Planta152, 93–100.
Lato, M., Brunelli, B., Giuffina, B., Mezzini, A., 1969: Thinlayer chromatography of sugars on silica gel impregnated with sodium acetate, monosodium phosphate and disodium phosphate. J. Chromat.39, 407–416.
Nakamura, S., Miki-Hirosige, H., Iwanami, Y., 1979: On the mechanisms of callose wall and callose plug formation in germinating pollen. Jap. J. Palynol.24, 33–44.
Northcote, D. H., Pickett-Heaps, J. D., 1966: A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem. J.98, 159–167.
Ott, D. W., Brown, R. M., 1974: Developmental cytology of the genusVaucheria. I. Organisation of the vegetative filament. Brit. Phycol. J.9, 11–126.
Pickett-Heaps, J. D., 1967: The use of autoradiography for investigating wall secretion in plant cells. Protoplasma64, 49–66.
Picton, J. M., Steer, M. W., 1981: Determination of secretory vesicle production rates by dictyosomes in pollen tubes ofTradescantia using cytochalasin D. J. Cell Sci.49, 261–272.
Roelofsen, P. A., Kreger, D. R., 1951: The submicroscopic structure of pectin in collenchyma cell walls. J. exp. Bot.2, 332–343.
Rosen, W. G., Gawlick, S. R., 1966: Fine structure of lily pollen tubes following various fixation and staining procedures. Protoplasma61, 181–191.
— —,Dashek, W. V., Siegesmund, K. A., 1964: Fine structure and cytochemistry ofLilium pollen tubes. Amer. J. Bot.51, 61–71.
Ruchel, R., 1976: Sequential protein analysis from single identified neurons ofAplysia californica. A microelectrophoretic technique involving polyacrylamide gradient gels and isoelectric focusing. J. Histochem. Cytochem.24, 773–791.
Sassen, M. A., 1964: Fine structure ofPetunia pollen grain and pollen tube. Acta Bot. Neerl.13, 175–181.
Schaffner, W., Weissmann, C., 1973: A rapid, sensitive and specific method for the determination of protein in dilute solution. Anal. Biochem.56, 502–514.
Shivanna, K. R., Heslop-Harrison, J., 1981: Membrane state and pollen viability. Ann. Bot.47, 759–770.
Van der Woude, W. J., Morré, D. J., 1968: Endoplasmic reticulum-dictyosome-secretory vesicle associations in pollen tubes ofLilium longiflorum Thunb. Proc. Indiana Acad. Sci.77, 164–170.
— —,Bracker, C. E., 1971: Isolation and characterisation of secretory vesicles in germinated pollen ofLilium longiflorum. J. Cell Sci.8, 331–351.
Weisenseel, M. H., Nuccitelli, R., Jaffé, L. F., 1975: Large electrical currents traverse growing pollen tubes. J. Cell Biol.66, 556–567.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heslop-Harrison, J., Heslop-Harrison, Y. The growth of the grass pollen tube: 1. Characteristics of the polysaccharide particles (“P-particles”) associated with apical growth. Protoplasma 112, 71–80 (1982). https://doi.org/10.1007/BF01280217
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280217