Summary
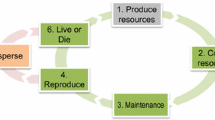
Dispersal as a means of escape from deteriorating habitats is of particular ecological relevance for organisms such as certain astigmatic mites that colonize habitats which vary unpredictably in space and time. The mites meet these ecological challenges by a facultative dispersal morph, the heteromorphic deutonymph, also called hypopus. The appearance or absence of hypopodes in natural populations is attributable to two fundamentally different, albeit interacting, causes. Genetic polymorphism for the propensity to induce a hypopus provides for heritable variation within the population and allows selection to favor or eliminate certain genotypes. The genotypic composition of a population reflects selection forces previously acting on the population. But it holds no predictive power. Rather, it adapts the population to cope with unpredictably varying living conditions because it ensures instantaneous fit of certain genotypes of the population (those displaying hypopus-free development) to favorable (moist) environmental conditions, and others (those expressing a hypopus) to detrimental (dry) conditions. In contrast, environmentally cued inducibility allows mites to anticipate food quality inasmuch as it allows each genotype of the population to adjust its development rapidly to impending adversity or benefit. Inducibility occurs by means of a developmental switching mechanism and leads either to a developmental pathway with a hypopus or else one without. The expression of a hypopus depends on interacting genetic and environmental (trophic) factors. High levels of additive genetic variation combine with considerable genetic-trophical interaction (comprising a threshold for phenotypic expression of the trait) to control hypopus induction. The results are consistent with a variable threshold whose level depends on diet quality. Different trophic conditions set the threshold at different points along the genetic scale resulting in different proportions of hypopus-forming and directly developing individuals within the population. The threshold, therefore, converts the concealed continuous genetic variation underlying the trait into a discontinuous response of the mite.
Similar content being viewed by others
References
Berthold, P. (1988) Evolutionary aspects of migratory behavior in European warblers.J. Evol. Biol. 1 195–209.
Berthold, P. (1991) Genetic control of migratory behaviour in birds.Trends Ecol. Evolut. 6 254–7.
Berthold, P., Wiltschko, W., Miltenberg, H. and Querner, U. (1990) Genetic transmission of migratory behavior into a nonmigratory bird population.Experientia 46 107–8.
Böttner, W. (1990) Untersuchungen zur Induktion des Hypopusstadiums bei der MilbeAcarus farris (Oudemans, 1905). Diplom-thesis, Institut für Angewandte Zoologie, Freie Universität Berlin.
Chmielewski, W. (1971) Morphology, biology and ecology ofCarpoglyphus lactis (L., 1758) (Glycyphagidae, Acarina).Prace Naukowe Instytutu Ochrony Roslin 13 63–166.
Chmielewski, W. (1973) A study on the influence of some ecological factors on the hypopus formation of stored product mites. InProceedings of the 3rd International Congress of Acarology, Prague, Czechoslovakia, 1971 (M. Daniel and B. Rosicky, eds) pp. 357–63. W. Junk, The Hague and Academia Publishing House of the Czechoslovak Academy of Sciences, Prague.
Chmielewski, W. (1977) Formation and importance of hypopus stage in the life of mites belonging to the superfamily Acaroidea.Prace Naukowe Instytutu Ochrony Roslin 19 5–94.
Corente, Ch. (1991) Getreideprodukte als trophische Faktoren für die Hypopus—Induktion des VorratsschädlingsLepidoglyphus destructor (Schrank) (Acari, Astigmata, Glycyphagidae). Diplom-thesis, Institut für Angewandte Zoologie, Freie Universität Berlin.
Cunnington, A.M. (1984) Resistance of the grain miteAcarus siro L. (Acarina: Acaridae) to unfavourable physical conditions beyond the limits of its development.Agric. Ecosyst. Environ. 11, 319–39.
den Boer, P.J. (1968) Spreading the risk and stabilization of animal numbers.Acta Biotheor. 18, 165–94.
Falconer, D.S. (1989)Introduction to Quantitative Genetics 3rd edn. Longman, London and Wiley, New York.
Fleurat-Lessard, F. (1975) Mise au point sur les acariens des pruneaux. Conditions de développement et de propagation desCarpoglyphus lactis L. (Acarida, Glycyphagidae).Rev. Zool. Agric. Pathol. Vég. 74, 121–38.
Gibbs, H.C. (1986) Hypobiosis in parasitic nematodes — an update.Adv. Parasitol. 25, 129–73.
Griffiths, D.A. (1966) Nutrition as a factor influencing hypopus formation in theAcarus siro species complex (Acarina: Acaridae).J. Stored Prod. Res. 1, 325–40.
Griffiths, D.A. (1969) The influence of dietary factors on hypopus formation inAcarus immobilis Griffiths (Acari, Acaridae). InProceedings of the 2nd International Congress of Acarology, Sutton Bonington, England, 1967 (G.O. Evans, ed.) pp. 419–32. Akadémiai Kiadó, Budapest.
Harrison, R.G. (1980) Dispersal polymorphism in insects.Annu. Rev. Ecol. Syst. 11, 95–118.
Hartl, D.L. and Clark, A.G. (1989)Principles of Population Genetics. Sinauer Associates, Sunderland, MA.
Hazel, W.D. and West, D.A. (1982) Pupal colour dimorphism swallowtail butterflies as a threshold trait: selection inEurytides marcellus (Cramer).Heredity 49, 295–301.
Hedrick, P.W. (1983)Genetics of Populations. Science Books International, Boston and Van Nostrand Reinhold Company, New York.
Houck, M.A. and O'Connor, B.M. (1991) Ecological and evolutionary significance of phoresy in the Astigmata.Annu. Rev. Entomol. 36, 611–36.
Knülle, W. (1963) Untersuchungen über den Einfluβ von Raumfeuchte, Temperatur und Lagerhöhe auf die Vermilbung von Trockenpflaumen.Z. Ang. Entomol. 52, 275–85.
Knülle, W. (1984) Water vapour uptake in mites and insects: an ecophysiological and evolutionary perspective. InAcarology VI, Vol. 1 (D.A. Griffiths and C.E. Bowman, eds) pp. 71–82. Ellis Horwood, Chichester.
Knülle, W. (1987) Genetic variability and ecological adaptability of hypopus formation in a stored product mite.Exp. Appl. Acarol. 3, 21–32.
Knülle, W. (1991) Genetic and environmental determinants of hypopus duration in the stored product miteLepidoglyphus destructor.Exp. Appl. Acarol. 10, 231–58.
Lawohnus, H. (1984) Induktion und Termination des Hypopus stadiums bei Populationen vonGlycyphagus destructor (Schrank, 1981) (Astigmata: Glycyphagidae) unterschiedlicher Standorte. Diplom-thesis. Institut für Angewandte Zoologie, Freie Universität Berlin.
Mayr, E. (1963)Populations, Species, and Evolution. Harvard University Press, Cambridge, MA.
Messina, F.J. (1990) Alternative life-histories inCallosobruchus maculatus: environmental and genetic bases. InBruchids and Legumes: Economics, Ecology and Coevolution. (K. Fujii, A.M.R. Gatehouse, C.D. Johnson, R. Mitchell and T. Yoshida, eds) pp. 303–15. Kluwer Academic, Dordrecht.
Naumann, M. (1986) Nahrungsart und Nahrungsqualität als Regulativ für die Inzidenz des Hypopusstadiums bei der HausmilbeGlycyphagus domesticus (De Geer 1778). Diplom-thesis, Institut für Angewandte Zoologie, Freie Universität Berlin.
Oboussier, H. (1939) Beiträge zur Biologie und Anatomie der Wohnungsmilben.Z. ang. Entomol. 26, 253–96.
Parkinson, C.L. (1990) Population increase and damage by three species of mites on wheat at 20°C and two humidities.Exp. Appl. Acarol. 8, 179–93.
Roff, D.A. (1986) The evolution of wing dimorphism in insects.Evolution 40, 1009–20.
Schneider, A. (1992) Ökologische Untersuchungen zur Induktion und Termination des Hypopusstadiums beiAcarus immobilis Griffiths (Acari, Astigmata, Acaridae). Diplom-thesis, Institut für Angewandte Zoologie, Freie Universität Berlin.
Sims, S.R. (1983) The genetic and environmental basis of pupal colour dimorphism inPapilio zelicaon (Lepidoptera: Papilionidae).Heredity 50, 159–68.
Stearns, S.C. (1976) Life history tactics: a review of the ideas.Q. Rev. Biol. 51, 3–47.
Stern, C. (1958) Selection for subthreshold differences and the origin of pseudoexogenous adaptations.Am. Nat. 92, 313–16.
Takafuji, A., So, P.-M. and Tsuno, N. (1991) Inter-and intra-population variations in diapause attribute of the two-spotted spider mite,Tetranychus urticae Koch, in Japan.Res. Popul. Ecol. 33, 331–44.
Tauber, C.A. and Tauber, M.J. (1987) Inheritance of seasonal cycles inChrysoperla (Insecta: Neuroptera).Genet. Res. Cam. 49, 215–23.
Tauber, M.J., Tauber, C.A. and Masaki, S. (1986)Seasonal Adaptations of Insects. Oxford University Press, New York and Oxford.
Treat, A.E. (1975)Mites of Moths and Butterflies. Comstock Publishing Associates, Ithaca and London.
Vitzthum, H. (1940) Die Deutonymphe vonCarpoglyphus lactis (L. 1763) (Acari, Tyroglyphidae).Zool. Anz. 129, 197–201.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knülle, W. Expression of a dispersal trait in a guild of mites colonizing transient habitats. Evol Ecol 9, 341–353 (1995). https://doi.org/10.1007/BF01237758
Issue Date:
DOI: https://doi.org/10.1007/BF01237758