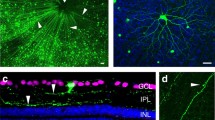
Summary
We have examined the ability of axotomized retinal ganglion cells in adult hamsters, to regenerate axons into a peripheral nerve graft attached to the optic nerve and the expression of GAP-43 by these neurons. We also examined the effect on these events of transplanting a segment of peripheral nerve to the vitreous body. The left optic nerves in three groups of hamsters were replaced with a long segment of peripheral nerve attached to the proximal stump of the optic nerve ∼2 mm from the optic disc to induce regeneration of retinal ganglion cells into the peripheral nerve. An additional segment of peripheral nerve was transplanted into the vitreous of the left eye in the second group. The animals from the first and second groups were allowed to survive for 1–8 weeks and the number of regenerating retinal ganglion cells was determined by applying the retrograde tracer, Fluoro-Gold to the peripheral nerve graft and the expression of GAP-43 was studied by immunocytochemistry in the same retinas. As a control, a segment of optic nerve was transplanted into the vitreous body of the left eye in the third group of hamsters. These animals were allowed to survive for 4 weeks and the number of regenerating retinal ganglion cells was counted as in Groups 1 and 2. The percentages of the regenerating retinal ganglion cells which also expressed GAP-43 were very high at all time points in Group 1 (with no intravitreal peripheral nerve) and Group 2 (with intravitreal peripheral nerve) and at 4 weeks for the Group 3 (with intravitreal optic nerve) animals. In addition, the number of regenerating retinal ganglion cells, the number of retinal ganglion cells expressing GAP-43 and the number of regenerating retinal ganglion cells which also expressed GAP-43 were much higher in Group 2 than in Group 1 at all the time points and it was also much higher in Group 2 than in Group 3 at 4 weeks whereas there was no significant difference between the results from Groups 1 and 3 at 4 weeks. These data suggested that there was a close correlation between the number of the axotomized retinal ganglion cells regenerating axons into the peripheral nerve graft attached to the optic nerve and the expression of GAP-43. In addition, the intravitreal peripheral nerve, probably by releasing various neurotrophic factors and by acting synergistically, can enhance the expression of GAP-43 in some of the axotomized retinal ganglion cells and promote the regeneration of retinal ganglion cells into the peripheral nerve graft.
Similar content being viewed by others
References
Aguayo, A. J. (1993) Regrowth and reconnection of severed axons in the visual system of adult mammals. InRegeneration and Plasticity in the Mammalian Visual System (edited byLam, D. M. K. &Bray M. B.) pp. 233–40. Cambridge: The MIT Press.
Aigner, L. &Caroni, P. (1993) Depletion of 43-kD growth-associated protein in primary sensory neurons leads to diminished formation and spreading of growth cones.Journal of Cell Biology 123, 417–29.
Benowitz, L. I. &Routtenberg, A. (1987) A membrane phosphoprotein associated with neural development, axonal regeneration, phospholipid metabolism, and synaptic plasticity.Trends in Neurosciences 10, 527–32.
Berry, M., Rees, L. &Sievers, J. (1986) Unequivocal regeneration of rat optic nerve axons into sciatic nerve isografts. InNeural Transplantation and Regeneration, (edited byDas, G. D. &Wallace, R. B.) pp. 63–79. New York: Springer-Verlag.
Campbell, G., Anderson, P. N., Turmaine, M. &Lieberman, A. R. (1991) GAP-43 in the axons of mammalian CNS neurons regenerating into peripheral nerve grafts.Experimental Brain Research 87, 67–74.
Carmignoto, G., Maffei, L., Candeo, P., Canella, R. &Comelli, C. (1989) Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section.Journal of Neuroscience 9, 1263–72.
Cho, E. Y. P. &So, K.-F. (1992) Characterization of the sprouting response of axon-like processes from retinal ganglion cells after axotomy in adult hamsters: a model using intravitreal implantation of a peripheral nerve.Journal of Neurocytology 21, 589–603.
Cho, K. S., Shen, L., So., K.-F. &Chung, S. K. (1994) Intravitreal transplantation of a peripheral nerve enhances c-jun expression in a subpopulation of axotomized retinal ganglion cells in hamsters.Proceedings of the 5th International Symposium on Neural Transplantation, Chatenay-Malatry, Paris, France, 056.
De Graan, P. N. E., Van Hoof, C. O. M., Tilly, B. C., Oestricher, A. B., Schotman, P. &Gispen, W. H. (1985) Phosphoprotein B-50 in nerve growth cones from fetal rat brain.Neuroscience Letters 61, 235–41.
Doster, S. K., Lozano, A. M., Aguayo, A. J. &Willard, M. B. (1991) Expression of the Growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury.Neuron 6, 635–47.
Federoff, H. J., Grabczyk, E. &Fishman, M. C. (1988) Dual regulation of GAP-43 gene expression by nerve growth factor and glucocorticoids.Journal of Biological Chemistry 263, 19290–5.
Heuman, R., Korsching, S., Bandtlow, C. &Thoenen, H. (1987) Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection.Journal of Cell Biology 104, 1623–31.
Kapfhammer, J. P. &Schwab, M. E. (1994) Inverse patterns of myelination and GAP-43 expression in the adult CNS: neurite growth inhibitors as regulators of neuronal plasticity?Journal of Comparative Neurology 340, 194–206.
Lau, K. C., So, K.-F. &Tay, D. (1994) Intravitreal transplantation of a segment of peripheral nerve enhances axonal regeneration of retinal ganglion cells following distal axotomy.Experimental Neurology 128, 211–15.
Lehwalder, D., Jeffrey, P. L. &Unsicker, K. (1989) Survival of purified embryonic chick retinal ganglion cells in the presence of neurotrophic factors.Journal of Neuroscience Research 24, 329–37.
Lei, J. L., Lau, K. C., Cho, E. Y. P. &So, K.-F. (1991) Morphological changes of retinal ganglion cells following intravitreal transplantation of a segment of peripheral nerve, December, 1991.Proceeding of the Fourth International Symposium on Neural Regeneration, The Asilomar Conference Center, Pacific Grove, California, USA, p. 41.
Lei, J. I., Lau, K. C., So, K.-F., Cho, E. Y. P. &Tay, D. (1995) Morphological plasticity of axotomized retinal ganglion cells following intravitreal transplantation of a peripheral nerve segment.Journal of Neurocytology 24, 497–507.
Maffei, L., Carmignoto, G., Perry, V. H., Candeo, P. &Ferrari, G. (1990) Schwann cells promote the survival of rat retinal ganglion cells after optic nerve section.Proceedings of the National Academy of Science (USA) 87, 1855–9.
Mansour-Robaey, S., Clarke, D. B., Wang, Y.-C., Bray, G. M. &Aguayo, A. J. (1994) Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells.Proceedings of the National Academy of Science (USA) 91, 1632–6.
Mayhew, T. M. (1992) A review of recent advances in stereology for quantifying neural structure.Journal of Neurocytology 21, 313–28.
McKerracher, L., David, S., Jackson, D. L., Kottis, V., Dunn, R. J. &Braun, P. E. (1994) Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth.Neuron 13, 805–11.
Mey, J. &Thanos, S. (1993) Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult ratsin vivo.Brain Research 602, 304–17.
Meyer, M., Matsuoka, I., Wetmore, C., Olson, L. &Thoenen, H. (1992) Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA.Journal of Cell Biology 119, 45–54.
Mitsumoto, H., Ikeda, K., Kinkosz, B., Cedarbaum, J. M., Wong, V. &Lindsay, R. M. (1994) Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF.Science 265, 1107–10.
Mukhopadhyay, G., Doherty, P., Walsh, F. S., Crocker, P. R. &Filbin, M. T. (1994) A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration.Neuron 13, 757–67.
Ng, T. F., So, K.-F. &Chung, S. K. (1992) Enhanced expression of GAP-43 in retinal ganglion cells by an intravitreous peripheral nerve graft after intraorbital axotomy in hamsters.Abstracts Society for Neuroscience 22, 426.
Ng, T. F., So, K.-F. &Chung, S. K. (1993) Intravitreal implantation of a peripheral nerve graft enhances expression of GAP-43 in retinal ganglion cells following axotomy.Neuroscience Letters Supplement 44, S30.
Richardson, P. M., Issa, V. M. K. &Shemie, S. (1982) Regeneration and retrograde degeneration of axons in the rat optic nerve.Journal of Neurocytology 11, 949–66.
Schwab, M. E., Kapfhammer, J. P. &Bandtlow, C. E. (1993) Inhibitors of neurite growth.Annual Review of Neuroscience 16, 565–95. New York: Raven Press.
Sendtner, M., Stockli, K. A. &Thoenen, H. (1992) Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration.Journal of Cell Biology 118, 139–48.
Skene, J. H. P. (1992) Retrograde pathways controlling expression of a major growth cone component in the adult CNS. InThe Nerve Growth Cone (edited byLetourneau, P. C., Kater, S. B. &Macagno, E. R.) pp 463–75. New York: Raven Press.
Skene, J. H. P., Jacobson, R. D., Snipes, G. J., Mcguire, C. B., Norden, J. J. &Freeman, J. A. (1986) A protein induced during nerve growth (GAP-43) is a major component of growth cone membranes.Science 233, 783–6.
So, K.-F. &Aguayo, A. J. (1985) Lengthy regrowth of cut axons from ganglion cells after peripheral transplantation into the retina of adult rats.Brain Research 328, 349–54.
So, K.-F., Xiao, Y. M. &Diao, Y. C. (1986) Effects on the growth of damaged ganglion cell axons after peripheral nerve transplantation in adult hamsters.Brain Research 337, 168–72.
So, K.-F., Ng, T. F., Chung, S. K., Lau, K. C. &Tay, D. (1994) Enhancement of axonal regeneration of retinal ganglion cells by a short segment of intravitreal peripheral nerve following proximal or distal replacement of the optic nerve graft.Proceedings of the 5th International Symposium on Neural Transplantation, Chatenay-Malatry, Paris, France, P192.
Stevenson, J. A. (1985) Growth of optic tract axons in nerve grafts in hamster.Experimental Neurology 87, 446–57.
Vaudano, E., Campbell, G., Anderson, P. N., Davies, A. P., Woolhead, C., Schreyer, D. J. &Leiberman, A. R. (1995) The effects of a lesion or a peripheral nerve graft on GAP-43 upregulation in the adult brain: anin situ hybridisation and immunocytochemical study.Journal of Neuroscience, in press.
Vidal-Sanz, M., Bray, G. M., Villegas-Perez, M. P., Thanos, S. &Aguayo, A. J. (1987) Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat.Journal of Neuroscience 7, 2894–909.
Villegas-Perez, M. P., Vidal-Sanz, M., Bray, G. M. &Aguayo, A. J. (1988) Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats.Journal of Neuroscience 8, 265–80.
Yankee, B. A., Benowitz, L. I., Villa-Komaroff, L. &Neve, R. L. (1990) Transfection of PC12 cells with the human GAP-43 gene: effects on neurite outgrowth and regeneration.Molecular Brain Research 7, 39–44.
Zeng, B. Y., Anderson, P. N., Campbell, G., Liberman, A. R. &Schreyer, D. J. (1992) GAP-43 in retinal ganglion cells of the adult rat after intraorbital and intracranial axotomy.Neuroscience Letters Supplement 42, S19.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ng, T.F., So, KF. & Chung, S.K. Influence of peripheral nerve grafts on the expression of GAP-43 in regenerating retinal ganglion cells in adult hamsters. J Neurocytol 24, 487–496 (1995). https://doi.org/10.1007/BF01179974
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01179974