Summary
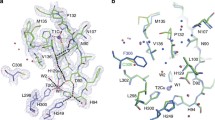
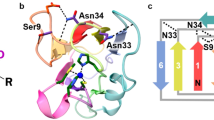
Biological electron transfer is not well understood. The question is addressed in this contribution with reference to the so-called blue copper proteins, each of which has a single copper atom at its active centre. The redox activity (as probed by the electron self exchange reaction) of the Cu centre seems not to be affected. The electron self exchange reaction is known to proceed through His-117, and the hydrophobic patch is most important in the formation of the azurin/azurin encounter complex. Ph effects have not been observed on the three-dimensional structure ofA. denitrificans azurin, which may indicate that if present at all these have no direct physiological implications. Mutants are in process of construction.
Similar content being viewed by others
References
Adman ET (1986) Structure and function of small blue copper proteins. In: Harrison PM (ed) Topics in Molecular and structural biology. Metalloproteins. MacMillan, New York, vol. 1, pp 1–42
Adman ET, Canters GW, Hill HAO, Kitchen NA (1982) The effect of pH and temperature on the structure of the active site of azurin fromPseudomonas aeruginosa. FEBS Lett 143:287–292
Anthony C (1989) Quinoproteins in C1-dissimilation by bacteria. In: Jongejan JA, Duine JA (eds) PQQ and quinoproteins. Kluwer, Dordrecht, pp 1–11
Antonini E, Finazzi-Agrò A, Avigliano L, Guerrieri P, Rotilio G, Mondovi B (1970) Kinetics of electron transport between azurin and cytochromec 551 fromPseudomonas. J Biol Chem 245:4847–4856
Baker EN (1988) Structure of azurin fromAlcaligenes denitrificans Refinement at 1.8 Å resolution and comparison of the two crystallographically independent molecules. J Mol Biol 203:1071–1095
Brill AS (1977) Transition metals in chemistry, Springer-Verlag, New York, pp 40–80
Canters GW (1987a) The azurin gene fromPseudomonas aeruginosa codes for a pre-protein with a signal peptide. FEBS Lett 212:168–172
Canters GW (1987b) Cloning and expression of a bacterial blue copper protein. Reel Trav Chim Pays-Bas 106:366–367
Corin AF, Bersohn R, Cole PE (1983) pH dependence of the reduction-oxidation reaction of azurin with cytochromec 551: role of histidine-35 of azurin in electron transport. Biochemistry 22:2032–2038
Farver O, Pecht I (1981) Electron transfer processes of blue copper proteins. In: Spiro TG (ed) Copper proteins. Wiley, New York, pp 161–192
Groeneveld CM, Canters GW (1988) NMR study of structure and et mechanism ofPseudomonas aeruginosa. J Biol Chem 263:167–173
Groeneveld CM, Aasa R, Reinhammar B, Canters GW (1987) EPR of azurins fromPseudomonas aeruginosa andAlcaligenes denitrificans demonstrates pH dependence of the copper-site geometry inPseudomonas aeruginosa protein. J Inorg Biochem 31:143–154
Groeneveld CM, Ouwerling MC, Erkelens C, Canters GW (1988)1H nuclear magnetic resonance study of the protonation behaviour of the histidine residues and the electron self-exchange reaction of azurin fromAlcaligenes denitrificans. J Mol Biol 200:189–199
Guss JM, Harrowell PR, Murata M, Norris VA, Freeman HC (1986) Crystal structure analysis of reduced poplar plastocyanin at six pH values. J Mol Biol 1923:361–387
Hill HAO, Smith BE (1979) Characteristics of azurin fromPseudomonas aeruginosa via 270-MHz1H nuclear magnetic resonance spectroscopy. J Inorg Biochem 11:79–93
Hoitink CWG, Woudt LP, Turenhout JCM, Van de Kamp M, Canters GW (1990) Isolation and sequencing of theAlcaligenes denitrificans azurin-encoding gene: comparison with the genes encoding blue copper proteins fromPseudomonas aeruginosa andA. faecalis. Gene, in the press
Mitchell P (1987) A new redox loop formality involving metalcatalysed hydroxide-ion translocation A hypothetical Cu loop mechanism for cytochrome oxidase. FEBS Lett 222:235–245
Mitra S, Bersohn R (1982) Proton NMR of the histidines of azurin fromAlcaligenes faecalis: linkage of histidine-35 with redox kinetics. Proc Natl Acad Sci USA 79:6807–6811
Palmer G (1987) Cytochrome oxidase: a perspective. Pure Appl Chem 59:749–758
Patterson Jr A, Ettinger R (1969) Nuclear magnetic resonance studies of the carbon dioxide-water equilibrium. Z Elektrochem 64:98–110
Rosen P, Segal M, Pecht I (1981) Electron transfer between azurin fromAlcaligenes faecalis and cytochromec 551 fromPseudomonas aeruginosa. Eur J Biochem 120:339–344
Ryden L, Lundgren J-O (1976) Homology relationships among the small blue proteins. Nature 261:344–346
Silvestrini MC, Brunori M, Wilson MT, Darley-Usmar VM (1981) The electron transfer system ofPseudomonas aeruginosa: a study of the pH-dependent transitions between redox forms of azurin and cytochromec 551. J Inorg Biochem 14:327–338
Tobari J, Harada Y (1981) Amicyanin: an electron acceptor of methylamine dehydrogenase. Biochem Biophys Res Commun 101:502–508
Ugurbil K, Norton RS, Allerhand A, Bersohn R (1977) Studies of individual carbon sites of azurin fromPseudomonas aeruginosa by natural-abundance carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry 16:866–894
Vallee BL, Williams RJP (1968) Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci USA 59:498–505
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Canters, G.W., Lommen, A., van de Kamp, M. et al. Structure and reactivity of type-I copper sites. Biol Metals 3, 67–72 (1990). https://doi.org/10.1007/BF01179505
Issue Date:
DOI: https://doi.org/10.1007/BF01179505