Summary
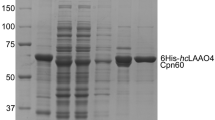
Lysine N6-hydroxylase was isolated as a soluble enzyme from the supernatant after ultrasonication ofEscherichia coli strain EN222 which contained the structural gene on a multicopy plasmid (as described by Engelbrecht and Braun in 1986). The apoenzyme prepared by dialysis was purified by ammonium sulfate precipitation and fast protein liquid chromatography using Superose 12 and Mono Q columns. The molecular mass as determined by gel filtration was 200 kDa and 50 kDa by SDS/polyacrylamide gel electrophoresis. The enzyme binds 0.79 molecule FAD/50 kDa. The activity of the enzyme is strictly dependent on NADPH. Its properties are similar to other flavoprotein monooxygenases of the EC group 1.14.13.
Similar content being viewed by others
References
Akers HA, Neilands JB (1978) Biosynthesis of rhodotorulic acid and other hydroxamate-type siderophores. In: Gorrod JW (ed) Biological oxidation of nitrogen. Elsevier, Amsterdam. pp 429–436
Ames GFL (1974) Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. J Biol Chem 249:634–644
Chen Y-C J, Peoples OP, Walsh CT (1988)Acinetobacter Cyclohexanone monooxygenase: Gene cloning and sequence determination. J Bacteriol 170:781–789
Czaky T (1948) On the estimation of bound hydroxylamine in biological materials. Acta Chem Scand 2:450–454
Engelbrecht F, Braun V (1986) Inhibition of microbial growth by interference with siderophore biosynthesis. Oxidation of primary amino groups in aerobactin synthesis byEscherichia coli. FEMS Microbiol Lett 33:223–227
Emery T (1971) Hydroxamic acids of natural origin. Adv Enzymol 5:135–185
Gross R, Engelbrecht F, Braun V (1984) Genetic and biochemical characterization of the aerobactin synthesis operon on pColV. Mot Gen Genet 196:74–80
Gross R, Engelbrecht F, Braun V (1985) Identification of the genes and their polypeptide products responsible for aerobactin synthesis by pCoIV plasmids. Mot Gen Genet 201:204–212
Herrero M, Lorenzo V de, Neilands JB (1988) Nucleotide sequence of theiucD gene of the pCo1V-K30 aerobactin operon and topology of its product studied withphoA andlacZ gene fusions. J Bacteriol 170:56–64
Heydel P, Plattner H, Diekmann H (1987) LysineN-hydroxylase andN-acetyltransferase of the aerobactin system of pColV plasmids inEscherichia coli. FEMS Microbiol Lett 40:305–309
Laemmli UK, Favre M (1973) Maturation of the head of bacteriophage T4. J Mot Biol 80:575–599
Lorenzo V de, Bindereif A, Neilands JB (1986) Aerobactin biosynthesis and transport genes of plasmid ColV-K30 inEscherichia coli K-12. J Bacteriol 165:570–578
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–278
Massey V, Hemmerich P (1975) Flavin and pteridine monooxygenases. In: Boyer P (ed) The enzyme vol. 12, Academic Press, New York, pp 191–252
Murray GJ, Clark GED, Parniak MA, Viswanatha T (1977) Effect of metabolites on ɛ-N-hydroxylysine formation in cellfree extracts ofAerobacter aerogenes 62-1. Can J Biochem 55:625–629
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731
Parniak MA, Jackson GED, Murray GJ, Viswanatha T (1979) Studies on the formation ofN 6-hydroxylysine in cell-free extracts ofAerobacter aerogenes 62-1. Biochim Biophys Acta 569:99–108
Pfefferle P (1987) Untersuchungen zur Biosynthese von Aerobactin: Eigenschaften der Lysin-N 6-hydroxylase ausEscherichia coli GR111 and EN222. Dissertation Universität Hannover
Studier FW (1973) Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mot Biol 79:237–248
Tomlinson G, Cruickshank WH, Viswanatha T (1971) Sensitivity of substituted hydroxylamines to determination by iodine oxidation. Anal Biochem 44:670–679
Wierenga RK, Kalk KH, van der Laan JM, Drenth J, Hofsteenge J, Weijer WJ, Jekel PA, Beintema JJ, Müller F, van Berkel WJH (1982) The structure and action ofp-hydroxy-benzoate hydroxylase. In: Nozaki M, Yamamoto S, Ishimura Y, Coon MJ, Ernster L, Estabrook RW (eds) Oxygenases and oxygen metabolism. Academic Press, New York, pp 187–205
Wierenga RK, Drenth J, Schulz GE (1983) Comparison of the three-dimensional protein and nucleotide structure of the FAD-binding domain ofp-hydroxybenzoate hydroxylase with the FAD- as well as NADPH-binding domains of glutathione reductase. J Mol Biol 167:725–739
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Plattner, HJ., Pfefferle, P., Romaguera, A. et al. Isolation and some properties of lysineN 6-hydroxylase fromEscherichia coli strain EN222. Biol Metals 2, 1–5 (1989). https://doi.org/10.1007/BF01116193
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01116193