Abstract
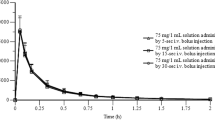
The kinetics of isosorbide dinitrate (ISDN) after i.v. administration and the absolute availability of an oral slow release preparation (SR) were studied in young healthy volunteers. ISDN and the 2- and 5-mononitrates of isosorbide (2-MN, 5-MN) were determined by GLC. After i.v. administration plasma levels of ISDN declined biexponentially and could be adequately described by an open two compartment body model. Distribution half-life was extremely rapid (2–5 min). Terminal disappearance had a half-life of 67 (62–75) min (mean, range). Total plasma clearance was 1.6 (1.2–2.2) litres · min−1, thus approaching liver blood flow. Nevertheless, absolute systemic availability (F) or oral ISDN amounted to 22% (16–29%). Assuming that oral ISDN is completely absorbed and blood levels do not exceed serum levels, an upper limit of hepatic clearance (liver blood flow 1.5 litres·min−1 · (1-F/100)) can be estimated, which is significantly smaller (p<0.05) than the measured clearance. This finding is best interpreted by assuming that ISDN is partly eliminated by extrahepatic routes, which is further substantiated by a different pattern of metabolites after i.v. and oral dosing. Whereas after i.v. administration more 2-MN is produced, 5-MN is the main metabolite after oral ISDN. Since the glutathione-S-transferases are found in the cytosol of most cells, it seems likely that other organs than the liver contribute to the metabolism of ISDN.
Similar content being viewed by others
References
D. T. Danahy and W. S. Aronow. Hemodynamic and antianginal effects of high dose oral isosorbide dinitrate after chronic use.Circulation 56:205–121 (1977).
J. A. Franciosa and J. N. Cohn. Hemodynamic responsiveness to short-and long-acting vasodilators in left ventricular failure.Am. J. Med. 65:126–131 (1978).
D. T. Danahy, D. T. Burwell and W. S. Aronow. Sustained hemodynamic and antianginal effect of high dose oral isosorbide dinitrate.Circulation 55:381–387 (1977).
G. Pierpout, K. A. Hale, and J. A. Franciosa. Effects of vasodilators on pulmonary hemodynamics and gas exchange in left ventricular failure.Am. Heart J. 99:208–213 (1980).
A. Distante, A. Maseri, and S. Severi. Management of vasospastic angina at rest with continuous infusion of isosorbide dinitrate.Am. J. Cardiol. 44:533–539 (1979).
T. Taylor, L. F. Chasseaud and E. Doyle. Pharmacokinetics of isosorbide dinitrate after intravenous infusion in human subjects.Biopharm. Drug Dispos. 1:149–156 (1980).
H. L. Fung, E. F. McNeff, and D. Ruggirello. Kinetics of isosorbide dinitrate and relationship to pharmacological effects.Br. J. Clin. Pharmacol. 11:579–590 (1981).
P. Needleman. Organic nitrate metabolism.Annu. Rev. Pharmacol. Toxicol. 16:81–93 (1976).
E. M. Johnson, A. B. Harkey, D. J. Blehm and P. Needleman. Clearance and metabolism of organic nitrates.J. Pharmacol. Exp. Ther. 182:56–62 (1972).
P. Needleman, S. Lang and E. M. Johnson. Organic nitrates: relationship between biotransformation and rational angina pectoris therapy.J. Pharmacol. Exp. Ther. 181:489–497 (1972).
D. O. Williams, W. J. Bommer and R. R. Miller. Hemodynamic assessment of oral peripheral vasodilator therapy in chronic congestive heart failure: prolonged effectiveness of isosorbide dinitrate.Am. J. Cardiol. 39:84–90 (1977).
P. A. Cassum and M. S. Roberts. Availability of isosorbide dinitrate, diazepam and chlormethiazole, from i.v. delivery systems.Eur. J. Clin. Pharmacol. 19:181–185 (1981).
H. Laufen, F. Scharpf and G. Bartsch. Improved method for the rapid determination of isosorbide dinitrate in human plasma and its application in pharmacokinetic studies.J. Chromatogr. 146:457–464 (1978).
L. B. Sheiner and S. Beal. Evaluation of methods for estimating population pharmacokinetic parameters I.J. Pharmacokin. Biopharm. 8:553–571 (1980).
L. B. Sheiner. ELSFIT. A program for the extended least squares fit to individual pharmacokinetic data. Users Manual. Division of Clinical Pharmacology, University of California, San Francisco (1980).
L. Z. Benet and R. L. Galeazzi. Noncompartmental determination of the steady state volume of distribution.J. Pharm. Sci. 68:1071–1074 (1979).
The Merck Index, ninth ed. Merck & Co., 1976.
U. Abshagen, G. Betzien, R. Endele and B. Kaufmann. Pharmacokinetics of intravenous and oral isosorbide-5-mononitrateEur. J. Clin. Pharmacol. 20:269–275 (1981).
L. F. Chasseaud, W. H. Down and R. K. Grundy. Concentrations of vasodilator isosorbide dinitrate and its metabolites in the blood of human subjects.Eur. J. Clin. Pharmacol. 8:157–160 (1975).
W. H. Down, L. F. Chasseaud and R. K. Grundy. Biotransformation of isosorbide dinitrate in humans.J. Pharm. Sci. 63:1147–1149 (1974).
S. F. Sisewine and H. W. Ruelius. Plasma concentrations and urinary excretion of isosorbide dinitrate and its metabolites in the dog.J. Pharmacol. Exp. Ther. 176:296–301 (1970).
S. Spörl-Radun, G. Belsien, B. Kaufmann, V. Liede and U. Abshagen. Effects and Pharmacokinetics of isosorbide dinitrate in normal man.Eur. J. Clin. Pharmacol. 18:237–244 (1980).
D. F. Assinder, L. F. Chasseaud and J. O. Hunter. Plasma concentrations of isosorbide dinitrate after oral administration of a sustained release formulation to human subjects.Drug Res. 27:156–158 (1977).
R. A. Morrison, H. F. Fung, D. Höhmann, T. Meinertz and E. Johnchen. Isosorbide dinitrate: pharmacokinetics after intravenous administration (letter).J. Pharm. Sci. 71:721–723 (1982).
W. H. Habig, M. J. Pabst and W. B. Jakoby. Glutathione-s-transferasesJ. Biol Chem. 249:7130–7139 (1979).
N. Kaplowitz, G. Clifton, J. Kuhlenkamp and J. D. Wallin. Comparison of renal and hepatic glutalhione-s-transferases of the rat.Biochem. J. 158:243–248 (1976).
L. M. Pinkus, J. N. Ketley and W. B. Jakoby. The glutathione-s-transferases as possible detoxification system of rat intestinal epithelium.Biochem. Pharmacol. 26:2359–2363 (1977).
C. Guthenberg, B. Mannervik. Purification of glutathione-s-transferases from rat lung by affinity chromatography.Biochem. Biophys. Res. Commun. 86:1304–1310 (1979).
J. R. Bend, M. Mukhtar, G. L. Faureman and I. P. Lee. Epoxide metabolizing enzyme activities in steroidogenic tissues in the rat.Fed. Proc. 36:961 (1977).
P. Kraus and H. D. Kloft. The activity of glutathione-s-transferases in various organs of the rat.Enzyme 25:158–169 (1980).
N. Kaplowitz, C. Spina, M. Graham and J. Kuhlenkamp. Glutathione-s-transferases in human lymphoid cell lines and fractionated peripheral leucocytes.Biochem. J. 169:465–470 (1978).
C. J. Marcus, W. M. Habig and W. B. Jakoby. Glutathione transferases from human erythrocytes.Arch. Biochem. Biophys. 188:287–293 (1978).
J. A. H. Campbell, N. M. Dass and R. E. Kirsch. Immunohistological localization of ligandin in human tissues.Cancer 45:503–510 (1980).
H. Mukhtar, C. E. Zoetemelk and A. J. Baars. Glutathione-s-transferase activity in human fetal and adult tissues.Pharmacology 22:322–329 (1981).
Author information
Authors and Affiliations
Additional information
This work was supported in part by a grant from the Swiss National Science Foundation and by a grant-in-aid of the Globopharm AG, Küsnacht, Switzerland.
Rights and permissions
About this article
Cite this article
Platzer, R., Reutemann, G. & Galeazzi, R.L. Pharmacokinetics of intravenous isosorbide-dinitrate. Journal of Pharmacokinetics and Biopharmaceutics 10, 575–586 (1982). https://doi.org/10.1007/BF01062541
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01062541