Abstract
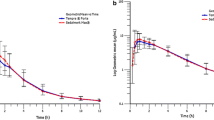
The biovailability of theophylline from alcoholic and aqueous oral solutions was compared to that from an intravenous dose in 12 normal adults. The alcoholic elixir surprisingly gave rise to a significantly greater (114 ±14%, mean±sd amount absorbed than did the intravenous dose. The aqueous solution (99±8%) and intravenous dose were statistically indistinguishable in this respect, and, furthermore, the extent of absorption from a 300-mg dose of the aqueous solution was 99±10% of that from a 500-mg dose, and not statistically different. The aqueous solution was thus employed in three subsequent studies as a standard with which to compare 13 different types of theophylline tablets, all marketed in the United States. Of the 13 tablets, eight showed bioavailability statistically distinguishable from that of the standard. Nevertheless, for only two tablets could it be claimed with 95% confidence that the bioavailability was less than 95%. For none can it be stated at this confidence level that the bioavailability is less than 90%. Bioavailability studies should include criteria of clinical significance in addition to criteria of statistical significance. Contrary to the usual rationale behind choice of a bioavailability standard, nine of the 12 uncoated tablets appeared to allow more rapid absorption of theophylline than did the standard oral solution, an aqueous syrup. Increasing the dose of syrup decreased the rate of theophylline absorption. Orally administered drug solutions may have properties more absorption rate limiting than the disintegration of many brands of tablet.
Similar content being viewed by others
References
R. I. Ogilvie. Clinical pharmacokinetics of theophylline.Clin Pharmacokin. 3:267–293 (1978).
L. Hendeles, M. Weinberger, and G. Johnson. Monitoring serum theophylline levels.Clin. Pharmacokin. 3:294–312 (1978).
C. W. Bierman. Theophylline in asthma.Pediatrics 58:623–635 (1976).
M. H. Jacobs, R. M. Senior, and G. Kessler. Clinical experience with theophylline: Relationship between dosage, serum levels and toxicity.J. Am. Med. Assoc. 235:1983–1986 (1976).
C. W. Zwillich, F. D. Sutton, T. A. Neff, W. M. Cohn, R. A. Matthay, and M. M. Weinberger. Theophylline induced seizures in adults.Ann. Intern. Med. 82:784–787 (1975).
P. R. Yarnell and N.-S. Chu. Focal seizures and aminophylline.Neurology 25:819–822 (1975).
S. P. Galant, S. A. Gillman, L. H. Cummins, P. P. Kozak, and J. J. Orcutt. Reliability of salivary theophylline as a guide to plasma theophylline levels.Am. J. Dis. Child. 131:970–972 (1977).
M. Danhof and D. D. Breimer. Therapeutic drug monitoring in saliva.Clin. Pharmacokin. 3:39–57 (1978).
K. C. Yeh and K. C. Kwan. A comparison of numerical integrating algorithms by trapezoidal, Lagrange and spline approximation.J. Pharmacokin. Biopharm. 6:79–98 (1978).
W. L. Chiou. Critical evaluation of the potential error in pharmacokinetic studies of using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve.J. Pharmacokin. Biopharm. 6:539–546 (1978).
RMEAS, A program for Repeated Measure Analysis of Variance, Version 3, 1977, A. Bostrom, Scientific Computing Services, University of California, San Francisco, Calif. 94143.
C. W. Dunnett. A multiple comparison procedure for comparing several treatments with a control.Am. Stat. Assoc. J. 50:1096–1121 (1955).
C. W. Dunnett. New tables for multiple comparisons with a control.Biometrics 20:482–491 (1964).
J. H. Zar.Biostatistical Analysis, Prentice-Hall, Englewood Cliffs, N.J. (1974) p. 198.
R. A. Upton, J.-F. Thiercelin, T. W. Guentert, S. M. Wallace, J. R. Powell, L. Sansom, and S. Riegelman. Intraindividual variability in theophylline pharmacokinetics. Statistical verification in 39 of 60 healthy young adults. Submitted toJ. Pharmacokin. Biopharm.
L. B. Sheiner, R. A. Upton, J.-F. Thiercelin, and S. Riegelman. Intraindividual variability in drug pharmacokinetics and its relevance to bioavailability testing. Submitted toJ. Pharmacokin. Biopharm.
W. J. Westlake. Use of statistical methods in evaluation ofin vivo performance of dosage forms.J. Pharm. Sci. 62:1579–1589 (1973).
M. Fixley, D. D. Shen, and D. L. Azarnoff. A comparison of the oral absorption of a theophylline elixir and two combination theophylline tablets to intravenous aminophylline.Ann. Rev. Resp. Dis. 115:955–962 (1977).
J. Caldwell, R. Lancaster, T. J. Monks, and R. L. Smith. The influence of dietary methylxanthines on the metabolism and pharmacokinetics of intravenously administered theophylline.Br. J. Clin. Pharmacol. 4:637p-638p (1977).
E. Ginchansky and M. Weinberger. Dose-dependent kinetics of theophylline disposition in asthmatic children.J. Pediatr. 91:820–824 (1977).
G. J. Kadlec, C. H. Jarboe, S. J. Pollard, and J. L. Sublett. Acute theophylline intoxication, biphasic first order elimination kinetics in a child.Ann. Allergy 41:337–339 (1978).
D. L. Spangler, D. D. Kalof, F. L. Bloom, and H. J. Wittig. Theophylline bioavailability following oral administration of six sustained-release preparations.Ann. Allergy 40:6–11 (1978).
M. Weinberger, L. Hendeles, and L. Bighley. The relation of product formulation to absorption of oral theophylline.New. Engl. J. Med. 229:852–857 (1978).
U.S. Department of Health, Education, and Welfare, Food and Drug Administration. Bioavailability and bioequivalence requirements.Fed. Register 42:1650 (January 7, 1977).
J. Cohen. Statistical Power Analysis for the Behavioral Sciences, Academic Press, New York, pp. 273–406.
P. G. Welling, L. L. Lyons, W. A. Craig, and G. A. Trochta. Influence of diet and fluid on bioavailability of theophylline.Clin. Pharmacol. Expl. Ther. 17:475–480 (1975).
R. A. Upton, J. R. Powell, T. W. Guentert, J.-F. Thiercelin, L. Sansom, P. E. Coates, and S. Riegelman. Evaluation of the absorption from some commercial enteric-release theophylline products.J. Pharmacokin. Biopharm. 8:151–164 (1980).
Author information
Authors and Affiliations
Additional information
This work was supported by FDA Contract No. 223-74-3145. Data management and analysis were achieved largely by the NIH-sponsored PROPHET system (ref.Proc. Natl. Comput. Conf. Exposition 43: 457, 1974). Dr. Guentert was supported by the Swiss National Science Foundation.
Rights and permissions
About this article
Cite this article
Upton, R.A., Sansom, L., Guentert, T.W. et al. Evaluation of the absorption from 15 commercial theophylline products indicating deficiencies in currently applied bioavailability criteria. Journal of Pharmacokinetics and Biopharmaceutics 8, 229–242 (1980). https://doi.org/10.1007/BF01059644
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059644