Abstract
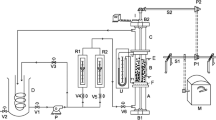
The electrochemical and thermal performance of a new thermogalvanic undivided flow cell using two aqueous electrolytes maintained at different temperatures between platinum electrodes is described. Measurements on the Fe(CN) 3−6 /Fe(CN) 4−6 redox system in NaOH medium yield a thermoelectric effect between −1.1 and −1.4mV/degree depending on the composition of the electrolytes. The influence of different parameters (temperature gradient, concentration of the electroactive species, fluid velocities and channel thickness) on the power output are determined. The experimental results show that electrical power is largely limited by charge and mass transfer overvoltages, but that it is possible to maintain the temperature gradient between the electrodes, i.e. the thermal boundary layers developing at the interface between the electrolytes do not reach the electrodes. A model based on the generalized Butler-Volmer kinetic equation is found to be in very good agreement with the experiments in terms of the current-voltage relationship and power output. This model is used for the prediction of the maximum electrochemical performance. From a practical point of view the efficiency of these devices remains very low due to the high thermal flux between the hot and cold electrolytes.
Similar content being viewed by others
Abbreviations
- C,C A,C B :
-
molar concentrations (mol m−3)
- C p :
-
mass heat capacity (J kg−1 K−1)
- D :
-
molecular diffusion coefficient (m2 s−1)
- d h :
-
hydraulic diameter of the cell (m)
- E :
-
equilibrium cell potential (V)
- F :
-
Faraday's constant=96487 C mol−1
- I :
-
cell intensity (A)
- l :
-
current density (A m−2)
- i L :
-
limiting current density (A m−2)
- i * :
-
dimensionless current density (defined in Equation 14)
- i 0 :
-
exchange current density (A m−2)
- k d :
-
mass transfer coefficient (m s−1)
- L :
-
length of channel (m)
- P :
-
electrical power (W)
- p :
-
specific electrical power (W m−2)
- Q m :
-
mass flowrate (kg s−1)
- R :
-
external electrical resistance (Ω)
- R i :
-
overall internal cell resistance (Ω or Ω m2)
- R m :
-
membrane specific resistance (Ω m2)
- R ′i :
-
ohmic internal resistance (Ω or Ω m2)
- Re :
-
Reynolds number
- Sh :
-
Sherwood number
- Sc :
-
Schmidt number
- t :
-
time (s)
- T :
-
temperature (K)
- u :
-
mean fluid velocity (m s−1)
- U c :
-
cell voltage (V)
- x :
-
parameter defined in Equation 14
- y :
-
coordinate normal to electrode (m)
- α:
-
electrochemical transfer coefficient (−)
- Δ:
-
half channel thickness (m)
- ΔT :
-
temperature difference (T c−T f) (K)
- γe :
-
number of electrons involved in the electrochemical reactions
- η:
-
overpotential (V)
- Φ:
-
thermal flux at the interface between the two flowing fluids (W)
- ϕ:
-
specific thermal flux (W m−2)
- λ:
-
parameter defined in Equation 8 (s−1/2)
- γ:
-
electrical conductivity (Ω m−1)
- A:
-
relative to species A
- a:
-
at the anode
- B:
-
relative to species B
- C:
-
relative to the hot electrode (anode)
- c:
-
at the cathode
- e:
-
at the electrode
- f:
-
relative to the cold electrode (cathode)
- opt:
-
optimal
- S:
-
in the bulk
References
W. Vielstich, ‘Fuel Cells’, Wiley Interscience, New York (1960) pp. 345–61.
A. J. De Bethune, T. S. Light and N. Swendeman,J. Electrochem. Soc. 106 (1959) 616.
R. Zito,A.I.A.A. Journal 1 (1963) 2133.
D. D. Macdonald, A. C. Scott and P. Wenteek,J. Electrochem. Soc. 110, (1963) 1618.
B. R. Sundheim, I. B. Cadoff and E. Miller, ‘Thermoelectrics and Devices’, Reinhold, New York (1960).
T. Wartanowicz,Adv. Energy Conversion 4 (1964) 149.
H. P. Meissner, D. C. White and G. D. Uhlrich,5 (1965) 205.
B. F. Markov and T. B. Kuzyakin,Russian Chemical Reviews 41 (1972) 250.
H. Reinhold,Z. Anorg. Allgem. Chem. 171 (1928) 181.
H. Reinhold and A. Blachny,Z. Elektrochem. 32 (1933) 290.
C. Wagner,Ann. Phys. 3 (1929) 629.
H. Holtan,Koningl. Nederland akad. Wetenschap. Proc. 1356 (1953) 498.
J. L. Weininger,J. Electrochem. Soc. 111, (1964) 769.
A. J. De Bethune,107 (1960) 829.
G. R. Salvi and A. J. De Bethune,108 (1961) 672.
J. N. Agar and W. J. Hamek, ‘The structure of electrolyte solutions’, Wiley & Sons, New York (1953) Chap. 13.
R. Gaboriaud and P. Letellier,J. Chim. Phys. 3 (1975) 357.
H. A. Liebhafsky, ‘Thermogalvanic cell’, US Patent (1959) 2882329.
B. H. Clampitt and D. E. German, US Patent (1966) 3253955.
B. W. Burrows, Proceedings of the 10th Intersociety Conversion Engineering conference (1975) 821.
,J. Electrochem. Soc. 123 (1976) 154.
I. M. Kolthoff and E. A. Pearson, ‘Stability of Potassium Ferrocyanide solutions. Industrial and Engineering Chemistry’,3 4 (1971) 381.
J. P. Bourne, P. Dell'ava, O. Dossenbach and T. Post,J. Chem. Eng. Data 30 (1985) 160.
N. B. Vargaftik, ‘Tables of the Thermophysical Properties of liquids and gases’, 2nd edition, Hemisphere Publishing, New York (1975).
D. Dobos, ‘Electrochemical Data’, Elsevier Scientific, New York (1975).
Y. S. Touloukian, P. E. Laley and S. C. Saxena, ‘Thermophysical Properties of Matter’, Vol. 3, Liley (1970).
E. W. Washburn, ‘International Critical Tables of Numeric Data, Physics, Chemistry and Technology’, Vol. 5, McGraw-Hill, New York (1929).
A. L. Horvath, ‘Handbook of Aqueous Solutions’, Wiley & Sons, New York (1985).
P. Bourret, private communication (1982).
J. M. Hornut, ‘Analyse Experimentale et théorique d'une pile thermogalvanique ou de concentration à convection forcée de deux liquides: Transfert de chaleur ou de matière à l'interface’, Ph. D. Thesis INPL-Nancy (1987).
K. J. Vetter, ‘Electrochemical Kinetics: Theoretical and Experimental Aspects’, Academic Press, New York (1967).
R. H. Norris and P. D. Streid,Trans. ASME 62 (1940) 525.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hornut, J.M., Storck, A. Experimental and theoretical analysis of a thermogalvanic undivided flow cell with two aqueous electrolytes at different temperatures. J Appl Electrochem 21, 1103–1113 (1991). https://doi.org/10.1007/BF01041456
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01041456