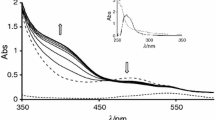
Abstract
Complexation of the zinc(II) ion with 2,2′-bipyridine (bpy) and 1,10-phenanthroline (phen) has been calorimetrically studied in 4-methylpyridine (4Me-py) containing 0.1 mol dm−3 (n-C4H9)4NClO4 as a constant ionic medium at 25°C. The formation of [ZnL]2+, [ZnL2]2+, and [ZnL3]2+ (L=bpy, phen), and their formation constants, reaction enthalpies and entropies were determined. Our EXAFS (extended X-ray absorption fine structure) measurements showed that the solvation structure of the manganese(II), cobalt(II), and nickel(II) ions is six-coordinate octahedral in 4Me-py and 3-methylpyridine (3Me-py), while that of the zinc(II) ion is four-coordinate tetrahedral in 4Me-py. Since [ZnL3]2+ is expected to have an octahedral structure, a tetrahedral-to-octahedral structural change should take place at a certain step of complexation. The thermodynamic parameters, especially reaction entropies, indicate that the structural change occurs at the formation of [Zn(bpy)2]2+ and [Zn(phen)]2+.
Similar content being viewed by others
References
S. Ahrland,Pure Appl. Chem. 51, 2019 (1979).
S. Ahrland,Pure Appl. Chem. 54, 1451 (1982).
S. Ahrland and S. Ishiguro,Inorg. Chim. Acta 142, 277 (1988).
S. Ahrland, S. Ishiguro, and I. Persson,Acta Chem. Scand. Ser. A 40, 418 (1986).
S. Ahrland and S. Balzano,Inorg. Chim. Acta 142, 285 (1988).
M. Kurihara, K. Ozutsumi, and T. Kawashima, (to be published).
M. Kurihara, K. Ozutsumi, and T. Kawashima,J. Chem. Soc. Dalton Trans. 3267 (1994).
V. Gutmann,The Donor-Acceptor Approach to Molecular Interactions (Plenum, New York, 1978).
Y. Abe, K. Ozutsumi, and S. Ishiguro,J. Chem. Soc. Faraday Trans. 1 85 3747 (1989).
Y. Abe and S. Ishiguro,J. Solution Chem. 20, 793 (1991).
S. Ishiguro, K. Ozutsumi, and H. Ohtaki,J. Chem. Soc. Faraday Trans. 1 84, 2409 (1988).
S. Ishiguro, K. Ozutsumi, and H. Ohtaki,Bull. Chem. Soc. Jpn. 60, 531 (1987).
K. Ozutsumi, M. Koide, H. Suzuki, and S. Ishiguro,J. Phys. Chem. 97, 500 (1993).
K. Ozutsumi, Y. Abe, R. Takahashi, and S. Ishiguro,J. Phys. Chem. 98, 9894 (1994).
H. Suzuki and S. Ishiguro,Netsu Sokutei 15, 152 (1988).
H. Ohtaki, T. Yamaguchi, and M. Maeda,Bull. Chem. Soc. Jpn. 49, 701 (1976).
M. Nomura, A. Koyama, and M. Sakurai,KEK Report 91-1 (National Laboratory for High Energy Physics, Tsukuba, Japan, 1991).
H. Suzuki and S. Ishiguro,Inorg. Chem. 31, 4178 (1992).
D. W. Marquardt,J. Soc. Ind. Appl. Math 11, 431 (1963).
F. J. Harris,Proc. IEEE 66, 51 (1978).
D. E. Sayers, E. A. Stern, and F. W. Lytle,Phys. Rev. Lett. 27, 1204 (1971).
E. A. Stern,Phys. Rev. B10, 3027 (1974).
E. A. Stern, D. E. Sayers, and F. W. Lytle,Phys. Rev. B11, 4836 (1975).
B. Lengeler and P. Eisenberger,Phys. Rev. B21, 4507 (1980).
B.-K. Teo and P. A. Lee,J. Am. Chem. Soc. 101, 2815 (1979).
S. Ishiguro, M. Miyauchi, and K. Ozutsumi,J. Chem. Soc. Dalton Trans. 2035 (1990).
T. Tamada, H. Suzuki, and S. Ishiguro, Abstracts of 42nd Symp. on Coordination Chemistry of Japan, (Nara, Japan, 1992) p. 36.
W. A. E. McBryde,A Critical Review of Equilibrium Data for Proton- and Metal Complexes of 1,10-Phenanthroline, 2,2′-Bipyridyl and Related Compounds, IUPAC Chemical Data Series, No. 17 (Pergamon, Oxford, 1978).
R. J. Doedens and L. F. Dahl,J. Am. Chem. Soc. 88, 4847 (1966).
G. Johansson and M. Sandström,Acta Chem. Scand. Ser. A 32, 109 (1978).
R. Åkesson, M. Sandström, C. Stålhandske, and I. Persson,Acta Chem. Scand. 45, 165 (1991).
R. D. Shannon,Acta Crystallogr. Sect. A 32, 751 (1976).
T. Yamaguchi and H. Ohtaki,Bull. Chem. Soc. Jpn. 51, 3227 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kurihara, M., Ozutsumi, K. & Kawashima, T. Complexation of the zinc(II) ion with 2,2′-bipyridine and 1,10-phenanthroline in 4-methylpyridine and solvation structure of the manganese(II), cobalt(II), nickel(II), and zinc(II) ions in 4-methylpyridine and 3-methylpyridine. J Solution Chem 24, 719–734 (1995). https://doi.org/10.1007/BF00973238
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00973238