Conclusions
-
1.
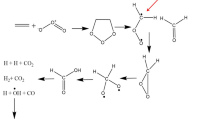
The thermal decomposition of N-nitro-N-bis-(2,2-dinitropropyl)amine in m-dinitrobenzene medium proceeds with autoacceleration. The first step of the process is monomolecular.
-
2.
The nature of the substituents in nitroamines does not influence the energy of dissociation of the N-NO2 bond.
-
3.
The increased reactivity of the compounds studied in comparison with N-nitrodialkylamines is due to the positive contribution to the entropy of activation, associated with steric hindrances to rotation around the N-NO2 bond.
-
4.
The enthalpy of formation of the CH3C(NO2)2CH2NCH2C(NO2)2CH3 radical was determined.
Similar content being viewed by others
Literature cited
J. M. Flournoy, J. Chem. Phys.,36, 1106 (1962).
B. L. Korsunskii and F. I. Dubovitskii, Dokl. Akad. Nauk SSSR,155, 402 (1964).
B. L. Korsunskii, F. I. Dubovitskii, and G. V. Sitonina, Dokl. Akad. Nauk SSSR,174, 1126 (1967).
B. L. Korsunskii, F. I. Dubovitskii, and E. A. Shurygin, Izv. Akad. Nauk SSSR, Ser. Khim., 1452 (1967).
F. M. Mukhametshin, A. L. Fridman, and A. D. Nikolaeva, Zh. Organ. Khimii,5, 928 (1970).
A. I. Gol'binder, Laboratory Studies for a Course on the Theory of Explosives [in Russian], Rosvuzizdat (1963).
L. M. Batuner and M. E. Pozin, Mathematical Methods in Chemical Technology [in Russian], Goskhimizdat (1960), p. 499.
A. B. Robertson, Trans. Faraday Soc.,45, 85 (1949).
Yu. Ya. Maksimov, The Theory of Explosives [in Russian], K. K. Andreev (editor), Vysshaya Shkola (1967), p. 73.
V. I. Pepekin, R. G. Gafurov, Yu. A. Lebedev, L. T. Eremenko, E. M. Sogomonyan, and A. Ya. Apin, Izv. Akad. Nauk SSSR, Ser. Khim., 318 (1973).
A. L. Fridman, F. M. Mukhametshin, and S. S. Novikov, Usp. Khimii,40, 64 (1971).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 8, pp. 1778–1781, August, 1974.
The authors would like to express their sincere gratitude to V. I. Pepekin for his discussion of the results obtained and to R.G. Gafurov for providing the sample of DNNA.
Rights and permissions
About this article
Cite this article
Korsunskii, B.L., Kiseleva, L.Y., Ramushev, V.I. et al. Kinetics of the thermal decomposition of bis-(2,2-dinitropropyl)-N-nitroamine. Russ Chem Bull 23, 1699–1701 (1974). https://doi.org/10.1007/BF00923191
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00923191