Abstract
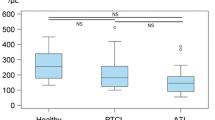
The aim of this study was to determine the effect of radiotherapy on peripheral blood natural killer (NK)-cell number and activity in 15 patients with cancer, prior to the commencement and at the completion of radiotherapy. The following observations were made. (i) Prior to radiotherapy NK activity could not be correlated with the stage of malignancy. (ii) In all patients with advanced disease and with subnormal baseline NK activity, the outcome of radiotherapy was unfavorable. (iii) Following radiotherapy to sites including the mediastinum, patients had decreased NK activity compared with those receiving treatment to other sites. This decrease was not related to the dose of radiotherapy or stage of malignancy. (iv) The tumor response was favorable in most patients whose NK activity decreased as a result of radiotherapy. (v) The decrease in NK activity may be associated with a decrease in the percentage of NK (N901) cells in the peripheral blood. The reduction in NK activity in those patients receiving mediastinal irradiation may be due to the large volume of blood which transits the field, so that the NK cells, or their more radiosensitive precursors, may be damaged and/or differentiation inhibited. Thus, these new observations show that radiotherapy does indeed affect the NK activity in cancer patients predominantly when the irradiation site includes the mediastinum.
Similar content being viewed by others
References
Hercend T, Reinherz EL, Meuller S, Schlossman SF, Ritz J: Phenotypic and functional heterogeneity of human cloned NK cell lines. Nature 301:158–160, 1982
Goldfarb RH, Herberman RB: Characteristics of natural killer cells and possible mechanisms for their cytotoxic activity.In Advances in Inflammation Research, Weissmann (ed.) New York, Raven Press, Vol 4, 1982, pp 45–72
Timonen T, Saksela E, Ranki A, Hayry P: Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol 48:133–148, 1979
Herberman RB: Natural killer cells. Hosp Pract 4:93–103, 1982
Herberman RB, Nunn ME, Holden HT, Larvin DH: Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer 16:230–239, 1975
Pross HF, Baines MG: Spontaneous human lymphocytemediated cytotoxicity against tumor target cells. I. The effect of malignant disease. Int J Cancer 18:593–604, 1976
Forbes JT, Greco FA, Oldham RK: Natural cell-mediated cytotoxicity in human patients.In Natural Cell-Mediated Immunity Against Tumours, RB Herberman (ed). New York, Academic Press, 1980, pp 1031–1046
Kadish AS, Doyle AT, Steinhauer EH, Ghossein NA: Natural cytotoxicity and interferon production in human cancer: Efficient natural killer activity and normal interferon production in patients with advanced disease. J Immunol 127:1817–1822, 1981
White D, Jones DB, Cooke T, Kirkham N: Natural killer (NK) activity in peripheral blood lymphocytes of patients with benign and malignant breast disease. Br J Cancer 46:611–616, 1982
Steinhauer EH, Doyle AT, Reed J, Kadish AS: Defective natural cytotoxicity in patients with cancer: Normal number of effector cells but decreased recycling capacity in patients with advanced disease. J Immunol 129:2255–2259, 1982
Stewart LD, Ades EW: Prospective study of natural cytotoxicity in peripheral blood of patients with nonlymphoid solid malignancies. Clin Immunol Immunopathol 3:78–86, 1984
Ortaldo JR, Phillips W, Wasserman K, Herberman RB: Effects of metabolic inhibitors on spontaneous and interferon boosted natural killer cell activity. J Immunol 125:1839–1844, 1980
Takasugi M, Ramseyer A, Takasugi J: Decline of natural nonselective cell-mediated cytotoxicity in patients with tumour progression. Cancer Res 17:413–418, 1977
Daynes RA, Bernhard EJ, Gurish MF, Lynch DH: Experimental photoimmunology: Immunologic ramifications of UV-induced carcinogenesis. J Invest Dermatol 77:77–85, 1981
Lynch DH, Gurish MF, Daynes RA: The effects of ultraviolet irradiation on the generation of anti-tumor cytotoxic effector cell responsesin vitro. J Immunol 127:1163–1168, 1981
Letvin NL, Fox IJ, Greene MI, Benacerraf B, German RN: Immunologic effects of whole body ultraviolet (UV) irradiation. II. Defect in splenic adherent cell antigen presentation for stimulation of T cell proliferation. J Immunol 125:1402–1404, 1980
Parkinson DR, Brighton, RP, Waksal SD: Altered natural killer cell biology in C57BL/6 mice after leukemogenic split-dose irradiation. J Immunol 126:1460–1464, 1981
Ahmann GB, Ballas ZK: Whole body ultraviolet (UV) irradiation decreases natural killer (NK) activity. Fed Proc 41:601, 1982
Lynch DH, Daynes RA: Evaluation of naturally occurring cell-mediated cytotoxic activity in normal and UV-irradiated mice. Transplantation 35(3):216–223, 1983
Markoe AM: The effects of combined radiation therapy and chemotherapy on the immune response. Prog Exp Tumour Res 25:219–228, 1980
Dean DM, Pross HF, Kennedy JC: Spontaneous human lymphocyte-mediated cytotoxicity against tumour target cells. III. Stimulatory and inhibitory effects of ionising radiation. Int J Rad Oncol Biol Phys 4:633–641, 1978
Blomgren H, Baral E, Edsmyr F, Strender L-E, Petrini B, Wasserman J: Natural killer activity in peripheral lymphocyte population following local radiation therapy. Acta Rad Oncol 19:139–143, 1980
Blomgren H, Strender L-E, Petrini B, Wasserman J: Changes of the spontaneous cytotoxicity of the blood lymphocyte population following local radiation therapy for breast cancer. Eur J Cancer Clin Oncol 18(7):637–643, 1982
Onsrud M, Thorsby E: Long-term changes in natural killer activity after external pelvic radiotherapy. Int J Rad Oncol Biol Phys 7:609–614, 1981
Kadish AS, Ghossein NA: Natural cytotoxicity in patients undergoing radiation therapy. Am J Clin Oncol (CCT) 6:53–59, 1983
Boyum A: Separation of leukocytes from blood and bone marrow. Scand J Clin Lab Invest 21 (Suppl 97):77–89, 1968
Lozzio CB, Lozzio BB: Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45:321–324, 1975
Pross HF, Baines MG: Studies of human natural killer cells. I.In vivo parameters affecting normal cytotoxic function. Int J Cancer 29:383–390, 1982
Kung PC, Goldstein GG, Reinherz EL, Schlossman SF: Monoclonal antibodies defining distinctive human T-cell surface antigens. Science 206:347–349, 1979
Griffin JD, Hercend T, Beveridge R, Schlossman SF: Characterisation of an antigen expressed by human natural killer cells. J Immunol 130:2947–2951, 1983
Gerson JM: Systemic andin situ natural killer activity in tumour-bearing mice and patients with cancer.In Natural Cell-Mediated Immunity Against Tumours, RB Herberman (ed) New York, Academic Press, 1980, pp 1047–1062
Vose BM, Vanky F, Fopp M, Klein E: Restricted autologous lymphocytotoxicity in lung neoplasia. Br J Cancer 38:375–381, 1978
Datta SK, Trentin JJ, Kurashige T: Natural killer cells in hamsters and their early augmentation and late suppression during tumour growth.In NK Cells and Other Natural Effector Cells, RB Herberman (ed). New York, Academic Press, 1982, pp 1207–1210.
Hurme M: Cell proliferation during the maturation of natural killer cells. Scand J Immunol 17:379–383, 1984
Cudkowicz G, Hochman PS: Do natural killier cells engage in regulated reactions against self to ensure homeostasis? Immunological Rev 44:13–41, 1979
Phillips WH, Ortaldo JR, Herberman RB: Selective depletion of human natural killer cells of monolayers of target cells. J Immunol 125:2322–2327, 1980
Salimonu LS, Ojo-Amize E, Williams AIO, Johnson AOK, Cooke AR, Adekunle FA, Alm GV, Wigzell H: Depressed natural killer cell activity in children with protein-calorie malnutrition. Clin Immunol Immunopathol 24:1–9, 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McGinnes, K., Florence, J. & Penny, R. The effect of radiotherapy on the natural killer (NK)-cell activity of cancer patients. J Clin Immunol 7, 210–217 (1987). https://doi.org/10.1007/BF00915726
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00915726