Abstract
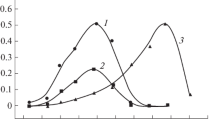
The [Co(BH)2(CN)(NO2)]− complex (BH2 = biacetyldioxime) has been obtained by means of an anatation reaction from the [Co(BH)2(NO2)(H2O)] nonelectrolyte with KCN in aqueous solution. The free acid H[Co(BH)2(CN)(NO2)]·4 H2O and 12 derivatives were obtained by double decomposition reactions. The thermal decomposition of some of these compounds was thermogravimetrically studied. Kinetics of the aquotization of the [Co(BH)2(CN)(NO2)]− was investigated in acid solution by following the change of the concentration of the free NO2 −-ions by means of a diazotation reaction. The kinetic parameters of the reaction are given.
Similar content being viewed by others
Literatur
P. R. Rây undN. K. Dutt, Z. anorg. allgem. Chem.234, 65 (1937).
P. R. Rây undT. Guptachaudhuri, Z. anorg. allgem. Chem.220, 154 (1934).
D. Banerjea undT. P. Das Gupta, J. inorg. nucl. Chem.29, 1021 (1967).
P. Tewari, R. W. Gawer, H. K. Wilcox undW. K. Wilmarth, J. Inorg. Chem.6, 611 (1967).
N. Maki, Nature [London]188, 224 (1960).
Cs. Várhelyi, I. Gănescu undL. Szotyori, Z. anorg. allgem. Chem.386, 232 (1971).
P. G. Lenhert undD. Crowfoot-Hodgin, Nature [London]192, 937 (1961).
A. V. Ablov undN. M. Samush, J. neorgan. Khim. [russ.]3, 1818 (1958).
A. V. Ablov, N. M. Samush undM. S. Popov, Dokl. Akad. Nauk SSSR.106, 665 (1956).
A. Nakahara, Bull. chem. Soc. Japan28, 473 (1955).
R. Blinc undD. Hadzi, J. Chem. Soc. [London]1958, 4536.
R. B. Penland, T. J. Lane undJ. V. Quagliano, J. Amer. Chem. Soc.78, 887 (1956).
W. P. Griffith undG. Wilkinson, J. Chem. Soc. [London]1959, 2757.
M. F. El-Sayed undR. K. Sheline, J. inorg. nucl. Chem.6, 187 (1958).
J. Zsakó, Z. Finta undCs. Várhelyi, Proc. 3rd Sympos. Coord. Chem. Debrecen (Hyngary), S. 333 (1970).
Z. Finta, J. Zsakó undCs. Várhelyi, Rev. Roum. Chim.16, 1731 (1971).
Cs. Várhelyi, J. Zsakó undZ. Finta, J. inorg. nucl. Chem.34, 2583 (1972).
Z. Finta, Cs. Várhelyi undE. Dakó, J. inorg. nucl. Chem., im Druck.
P. Griess, Ber. dtsch. chem. Ges.12, 428 (1879);L. Ilosway, Bull. soc. chim. France2, 347 (1889).
K. A. Pilkington undP. J. Staples, J. inorg. nucl. Chem.29, 1029 (1967).
Author information
Authors and Affiliations
Additional information
Mit 2 Abbildungen
Rights and permissions
About this article
Cite this article
Várhelyi, C., Finta, Z. & Kiss, S. Der Cyano-nitro-bis-biacetyldioximato-kobalt(III)-Komplex und seine Aquatisierungskinetik in saurem Medium. Monatshefte für Chemie 106, 1325–1332 (1975). https://doi.org/10.1007/BF00913606
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00913606