Summary
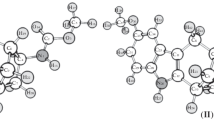
Addition of sulfur dichloride to tetrachlorocatechol-bisallylether (1) yields the 9- and 10-ring thia crown ether derivatives2 and3, respectively, together with the dithia-18-crown-6-ether4. The 10-membered ring compound3 represents the first thia macrocycle containing bothMarkovnikov andanti-Markovnikov constitution of the β-chloro-thio structural segments in the same molecule. By1H and13C NMR spectroscopy, equal amounts of two preferred conformers of the only isolated diastereomer of3 were observed at temperatures below −50°C. The signals were assigned to these conformers using COSY, HETCOR, and phase sensitive NOESY spectra at low temperatures. The preferred conformations and the relative configuration were determined using the different effects of γ gauche -and γ anti -positions in13C NMR chemical shifts and analyzing vicinal3 J H,H coupling constants. These results were confirmed by molecular mechanics calculations.
Zusammenfassung
Aus Tetrachlorbrenzcatechin-bisallylether (1) und Schwefeldichlorid wurden neben der 1,10-Dithia-dibenzo-18-krone-64 die 9- und 10-Ring-Derivate2 und3 erhalten. In der 10-Ring-Verbindung wurde erstmaligMarkovnikov- undanti-Markovnikov-Konstitution der β-Chlor-thio-Gruppierung in einem Thiamakrocyclus nebeneinander vorgefunden. Das isolierte Diastereomere von3 zeigt bei Temperaturen unter −50 °C zwei Konformere. Die Signalzuordnung war mit homo-und heteronuklearen COSY- sowie mit phasensensitiven NOESY-Spektren möglich. Mit Hilfe molekülmechanischer Rechnungen konnten aus den13C-NMR-Verschiebungen und den vicinalen H,H-Kopplungskonstanten die Vorzugskonformeren und die relative Konfiguration bestimmt werden.
Similar content being viewed by others
References
Heinicke J (1994) Untersuchungen zur Chemie und Stereochemie von Allylphenylether-SCl2-Additionsprodukten. Thesis, University of Leipzig
Kleinpeter E, Hollmann K, Mühlstädt M (1990) Z Chem30: 63
Stoss S, Schroth W, Kleinpeter E (1992) Magn Reson Chem30: 425
Stoss S, Kleinpeter E, Holdt HJ (1991) Magn Reson Chem29: 999
Kleinpeter E, Stoss S, Gäbler M, Schroth W (1989) Magn Reson Chem27: 676
Kleinpeter E, Gäbler M, Schroth W, Mattinen J, Pihlaja K (1988) Magn Reson Chem26: 387
Kleinpeter E, Gäbler M, Schroth W (1988) Magn Reson Chem26: 380
Kleinpeter E (1991) J Prakt Chem333: 817
Buchanan GW, Moghimi A, Bensimon C (1995) Can J Chem73: 100
Buchanan GW, Driega AB, Ratcliffe C (1993) Magn Reson Chem31: 1094
Nazhaoui M, Joly JPA, Jean-Claude BJ, Del Duca V, Aubry A, Boubouh M (1995) J Chem Soc Perkin Trans 1,22: 2919
Rossa L, Vögtle F (1983) Topics in Current Chem113: 1
Hisamoto H, Nakagawa E, Nagatsuka K, Abe Y, Sato S, Siswanta D, Suzuki K (1995) Anal Chem67: 1315
Dalley NK, Larson SB, Smith JS, Smith JS, Matheson KL, Izatt RM, Christensen JJ (1981) J Heterocycl Chem18: 463
Van Gunsteren WF, Berendsen HJC (1990) Angew Chem102: 1020
SYBYL: Molecular Modelling Software, Version 7.1 (1995) Tripos, Inc. St. Louis
Dietrich B, Viout P, Lehn JM (1993) Macrocyclic Chemistry: Aspects of Organic and Inorganic Supramolecular Chemistry. VCH Verlagsgesellschaft, Weinheim, p 201
Salvino JM, Seoane PR, Dolle RE (1993) J Comp Chem14: 438
Otten RHJM, van Ginnecken LPPP (1989) The Annealing Algorithm. Kluwer Academic Press, Boston
Pihlaja K, Kleinpeter E (1994) Carbon-13 NMR Chemical Shifts in Structural and Stereochemical Analysis. In: Marchand AP (ed) Methods in Stereochemical Analysis. VCH Verlagsgesellschaft, Weinheim New York Cambridge, p 58
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr.Rolf Borsdorf on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Meusinger, R., Heinicke, J., Möhle, K. et al. Conformational analysis of thia crown ether derivatives by NMR spectroscopy and molecular mechanics calculations. Monatsh Chem 127, 1145–1152 (1996). https://doi.org/10.1007/BF00844689
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00844689