Abstract
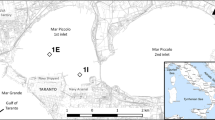
The early diagenetic environment of intertidal sandy sediments (sands) and muddy sediments (muds) is described and compared from two cores taken from an unpolluted part of the Manukau Harbour, New Zealand. Extraction techniques characterized the form of the trace elements (Fe, Mn, S, C, Pb, Zn, Cu) at different depths in the sediment. Dissolved forms of Fe, Mn, and S were measured in interstitial water. Nonresidual metal concentrations, humic acid, FeS, and FeS2 are an order of magnitude higher in the muds than in the sands because of dilution by unreactive sand particles. Muds contain a larger proportion of metals in the mobile fractions; exchangeable (Mn), carbonate (Mn, Fe, Zn), and easily-reducible oxide (Fe, Mn, Zn, Pb). This is due to greater surface area (for Mn adsorption); the favorable conditions for MnCO3, FeCO3, and FeS precipitation; and higher concentrations of easily reducible iron oxide and humic acid. Therefore, compared to the sands, muds are more important as reservoirs for toxic metals, both in terms of quantity and availability. At either site there was very little difference between the forms of Zn, Pb or Cu identified by sequential extraction as sediments changed from oxic to anoxic conditions. One reason for this is that the amounts and proportions of some of the important components that bind metals, viz., amorphous iron hydrous oxides, humic acids, and FeS2, do not change much. Other components that do change with redox conditions, for example, manganese phases and FeS, are only minor components of the sediment. Redox conditions, then, have relatively little effect on trace-metal partitioning in the sediment matrix of these unpolluted sediments.
Similar content being viewed by others
References
American Public Health Association (1989) Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association; American Water Works Association; Water Pollution Control Federation. 1467 pp
Baeyens W, Panutrakul S, Elskens M, Leermakers M, Navez J, and Monteny F (1991) Geochemical processes in muddy and sandy tidal flat sediments. Geo-Mar Lett 11:188–193
Belzile N, Lecomte P, and Tessier A (1989) Testing readsorption of trace elements during partial chemical extractions of bottom sediments. Environ Sci Technol 23:1015–1020
Berner RA (1971) Principles of sediment geochemistry. New York: McGraw-Hill. 240 pp
Boughriet A, Ouddane B, and Wartel M (1992) Electron spin resonance investigations of Mn compounds and free radicals in particles from the Seine river and its estuary. Mar Chem 37:149–169
Brown DS and Allison JD (1987) MINTEQA1, An equilibrium metal speciation model: Users manual. Athens, Georgia: US Environmental Protection Agency, EPA 600/3-87/012
Canfield DE (1989) Reactive iron in marine sediments. Geochim Cosmochim Acta 53:619–632
Canfield DE and Berner RA (1987) Dissolution and pyritization of magnetite in anoxic marine sediments. Geochim Cosmochim Acta 51:645–659
Canfield DE, Raiswell R, Westrich JT, Reaves CM and Berner RA (1986) The use of chromium reduction in the analysis of reduced inorganic sulphur in sediments and shales. Chem Geol 54:149–155
Cannelli E, Mitchell DG and Pause RW (1976) An improved determination of chemical oxygen demand in the water and wastes by a simplified acid dichromate digestion. Water Res 10:351–355
Chanton JP and Martens CS (1985) The effects of heat and stannous chloride addition on the active distillation of acid volatile sulphide from pyrite rich marine sediment samples. Biogeochemistry 1:375–383
Chester R, Thomas A, Lin FJ, Basaham AS and Jacinto G (1988) The solid state speciation of copper in surface water particulates and oceanic sediments. Mar. Chem 24:261–292
Davies-Colley RJ, Nelson PO, and Williamson KJ (1985) Sulfide control of cadmium and copper concentrations in anaerobic estuarine sediments. Mar Chem 16:173–186
De Mora SJ and Demeke G (1990) Chemical fractionation of lead in intertidal sediments from Manukau Harbour, Auckland, New Zealand. NZ J Mar Freshwater Res 24:569–575
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Carlson AR and Ankley GT (1992) Acid volatile sulfide predicts the acute toxicity of cadmium and nickel in sediments. Environ Sci Technol 26:96–101
Dolphin TJ (1992) Low amplitude multiple bar systems in an intertidal fetch limited environment. Unpublished MSc thesis. University of Auckland, 142 pp
Dyrssen D (1985) Metal complex formation in sulphidic seawater. Mar Chem 15:284–293
El Ghobary H and Latouche C (1986) Distribution of certain metals in lithochemical fractions of sediments from the Arcachon basin, south west France: Authigenic mineral formation and pollution. Chem Geol 54:295–309
Evans DW, Cutshall NH, Cross FA and Wolfe DA (1977) Manganese cycling in the Newport River Estuary, North Carolina. Estuarine Coastal Shelf Sci 5:71–80
Felmy AR, Girvin DC and Jenne EA (1984) MINTEQ. A computer program for calculating aqueous geochemical equilibria. Athens, Georgia: US Environmental Protection Agency, EPA 600/3-84/ 032
Folk RL (1986) Petrology of sedimentary rocks. Austin: University of Texas, 170 pp
Fox ME, Roper DR, and Thrush SF (1988) Organochlorine contaminants in the surficial sediments of Manukau Harbour, New Zealand. Mar Pollut Bull 19:333–336
Froelich PN, Klinkhammer GP, Bender ML, Luedtke NA, Heath GR, Cullen D, Dauphin D, Hammond D, Hartman B and Maynard V (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochim Cosmochim Acta 43:1075–1090
Gerringa LJA (1990) Aerobic degradation of organic matter and the mobility of Cu, Cd, Ni, Fe, and Mn in marine sediment slurries. Mar Chem 29:355–374
Gerringa LJA (1991) Mobility of Cu, Cd, Ni, Pb, Zn, Fe and Mn in marine sediment slurries under anaerobic conditions and at 20% air saturation. Neth J Sea Res 27:145–156
Gieskes JM and Rogers WC (1973) Alkalinity determinations in interstitial water of marine sediments. J Sediment Petrol 43:272–277
Glasby GP, Stoffers P, Walter P, Davis KR, and Renner RM (1988) Heavy-metal pollution in Manukau and Waitemata harbours, New Zealand. NZ J Mar Freshwater Res 22:595–611
Gratton Y, Edenborn HM, Silverberg N, and Sundby B (1990) Diagenesis in bioturbated sediments. Am J Sci 290:246–262
Hines ME, Lyons WB, Armstrong PB, Orem WH, Spencer MJ, Gaudette HE, and Jones GE (1984) Seasonal metal remobilization in the sediments of Great Bay, New Hampshire. Mar Chem 15:173–187
Hume TM, Fox ME, and Wilcock RJ (1989) Use of organochlorine contaminants to measure sedimentation rates in estuaries: A case study from the Manukau Harbour, New Zealand. J R Soc NZ 19:305–317
Kersten M and Forstner U (1986) Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments. Water Sci Technol 18:121–130
Kheboian C and Bauer CF (1987) Accuracy of selective extraction procedures for metal speciation in model aquatic sediments. Anal Chem 59:1417–1423
Luoma SN (1990) Processes affecting metal concentrations in estuarine and coastal marine sediments. In: Heavy metals in the marine environment. Furness RW and Rainbow PS (Eds), Boca Racton, Florida: CRC Press, pp 51–66
Martin JM, Nirel P, and Thomas AJ (1987) Sequential extraction techniques: promises and problems. Mar Chem 22:313–341
Morris AW, Mantoura RFC, Bale AJ, and Howland RJM (1978) Very low salinity regions in estuaries: Important sites for chemical and biological reactions. Nature 274:678–680
Nembrini GP, Rapin F, Garcia JI, and Forstner U (1982) Speciation of Fe and Mn in a sediment core of the Baie de VilleFrance (Mediterranean Sea, France), Environ Technol Lett 3:545–552
Orion Corporation (1978) Instrument manual sulphide ion electrode. Orion Corporation Inc
Panutrakul S and Baeyens W (1991) Behaviour of heavy metals in a mud flat of the Scheldt Estuary, Belgium. Mar Pollut Bull 22:128–134
Pickering RF (1986) Metal ion speciation—soils and sediments (a review). Ore Geol Rev 1:83–146
Price BN (1976) Chemical diagenesis in sediments. In: Riley JP and Chester R (Eds), Chemical Oceanography, Vol. 6. London: Academic Press. pp 1–58
Pridmore RD, Thrush SF, Hewitt JE, and Roper DS (1990) Macrobenthic community composition of six intertidal sandflats in the Manukau Harbour N.Z. NZ J Mar Freshwater Res 24:81–96
Rapin F, Nembrini GP, Garcia JI, and Forstner U (1983) Heavy metals in marine sediment phases determined by sequential chemical extraction and their interaction with interstitial water. Environ Technol Lett 4:387–396
Rapin F, Tessler A, Campbell PGC, and Carignan R (1986) Potential artifacts in the determination of metal partitioning in sediments by a sequential extraction procedure. Environ Sci Technol 20:836–840
Rendell PS, Batley GE, and Cameron AJ (1980) Adsorption as a control of metal concentrations in sediment extracts. Environ Sci Technol 14:314–318
Roper DS, Thrush SF, and Smith DG (1988) The influence of runoff on intertidal mudflat benthic communities. Mar Environ Res 26:1–18
Salomons W, de Rooij NM, Kerdijk H, and Bril J (1987) Sediments as a source for contaminants? Hydrobiology 149:13–30
Schoonen MAA and Barnes HL (1991) Reactions forming pyrite and marcasite from solution: II. Via FeS percursors below 100°C. Geochim Cosmochim Acta 55:1505–1514
Shannon RD and White JR (1991) The selectivity of a sequential extraction procedure for the determination of iron oxyhydroxides and iron sulfides in lake sediments. Biogeochemistry 14:193–208
Shaw TJ, Gieskes JM, and Jahnke RA (1990) Early diagenesis in differing depositional environments: The response of transition metals in pore water. Geochim Cosmochim Acta 54:1233–1246
Sorensen J and Jorgensen BK (1987) Early diagenesis in sediments from Danish coastal waters: Microbial activity and Mn-Fe-S geochemistry. Geochim Cosmochim Acta 51:1583–1590
Stumm W and Morgan JJ (1981) Aquatic chemistry. New York: John Wiley & Sons 780 pp
Suess E (1979) Mineral phases formed in anoxic sediments by microbial decomposition of organic matter. Geochim Cosmochim Acta 43:339–352
Watkinson JH, Lee A, and Lauren DR (1987) Measurement of elemental sulfur in soil and sediments: field sampling, sample storage, pretreatment, extraction and analysis by high performance liquid chromatography. Aust J Soil Res 25:167–178
Watson PG, Frickers PE, and Goodchild CM (1985) Spatial and seasonal variations in the chemistry of sediment interstitial waters in the Tamar estuary. Estuarine Coast Shelf Sci 21:105–119
Williamson RB, Blom A, Hume TM, Glasby GP, and Larcombe M (1992) Heavy metals in Manukau Harbour sediments. Hamilton, New Zealand: National Institute of Water and Atmospheric Research. 50 pp
Wilson DE (1980) Surface and complexation effects on the rate of Mn(II) oxidation in natural waters. Geochim Cosmochim Acta 44:1311–1317
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Williamson, R.B., Hume, T.M. & Mol-Krijnen, J. A comparison of the early diagenetic environment in intertidal sands and muds of the Manukau Harbour, New Zealand. Geo 24, 254–266 (1994). https://doi.org/10.1007/BF00767086
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00767086