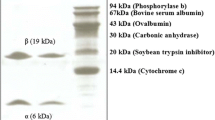
Abstract
The Orchidaceae speciesListera ovata andEpipactis helleborine contain two types of mannose-binding proteins. Using a combination of affinity chromatography on mannose-Sepharose-4B and ion exchange chromatography on a Mono-S column eight different mannose-binding proteins were isolated from the leaves ofListera ovata. Whereas seven of these mannose-binding proteins have agglutination activity and occur as dimers composed of lectin subunits of 11–13 kDa, the eighth mannose-binding protein is a monomer of 14 kDa devoid of agglutination activity. Moreover, the monomeric mannose-binding protein does not react with an antiserum raised against the dimeric lectin and, in contrast to the lectins, is completely inactive when tested for antiretroviral activity against human immunodeficiency virus type 1 and type 2. Mannose-binding proteins with similar properties were also found in the leaves ofEpipactis helleborine. However, in contrast toListera only one lectin was found inEpipactis. Despite the obvious differences in molecular structure and biological activities molecular cloning of different mannose-binding proteins fromListera andEpipactis has shown that these proteins are related and some parts of the sequences show a high degree of sequence homology indicating that they have been conserved through evolution.
Similar content being viewed by others
Abbreviations
- EHMBP:
-
Epipactis helleborine mannose-binding protein
- LOMBP:
-
Listera ovata mannose-binding protein
References
Van Damme EJM, Allen AK, Peumans WJ (1987)FEBS Lett 215:140–4.
Van Damme EJM, Allen AK, Peumans WJ (1988)Physiol Plant 73:52–7.
Van Damme EJM, Goldstein IJ, Peumans WJ (1991)Phytochemistry 30:509–14.
Van Damme EJM, Allen AK, Peumans WJ (1987)Plant Physiol 85:566–9.
Van Damme EJM, Smeets K, Torrekens S, Van Leuven F, Peumans WJ (1994)Eur J Biochem 221:769–77.
Saito K, Komae A, Kakuta M, Van Damme EJM, Peumans WJ, Goldstein IJ, Misaki A (1993)Eur J Biochem 217:677–81.
Balzarini J, Schols D, Neyts J, Van Damme E, Peumans W, De Clercq E (1991)Antimicrob Agents Chemother 35:410–16.
Balzarini J, Neyts J, Schols D, Hosoya M, Van Damme E, Peumans W, De Clercq E (1992)Antiviral Res 18:191–207.
Popovic M, Sarngadharan MG, Read E, Gallo RC (1984)Science 224:497–500.
Clavel F, Guétard D, Brun-Vézinet F, Chamaret S, Rey M-A, Santos-Ferreira MO, Laurent AG, Dauget C, Katlama C, Rouzioux C, Klatzmann D, Champalimaud JL, Montagnier L (1986)Science 233:343–46.
Bradford MM (1976)Anal. Biochem. 72:248–54.
Laemmli UK (1970)Nature 227:680–5.
Van Damme EJM, Goldstein IJ, Vercammen G, Vuylsteke J, Peumans WJ (1992)Physiol Plant 86:245–52.
Van Damme EJM, Smeets K, Torrekens S, Van Leuven F, Goldstein IJ, Peumans WJ (1992)Eur J Biochem 206:413–20.
Balzarini J, Naesens L, Herdewijn P, Rosenberg I, Holy A, Pauwels R, Baba M, Johns DG, De Clercq E (1989)Proc Natl Acad Sci USA 86:332–6.
Balzarini J, Naesens L, Slachmuylders J, Niphuis H, Rosenberg I, Holy A, Schellekens H, De Clercq E (1991)AIDS 5:21–8.
Finkelstein RR, Crouch ML (1986)Plant Physiol 81:907–12.
Siflow CD, Hammett JR, Key JL (1979)Biochemistry 18:2725–31.
Wadsworth GJ, Redinbough MG, Scandalios JG (1988)Anal Biochem 172:279–83.
Van Damme EJM, Kaku H, Perini F, Goldstein IJ, Peeters B, Yagi F, Decock B, Peumans WJ (1991)Eur J Biochem 202:23–30.
Mierendorf RC, Pfeffer D (1987)Meth Enzymol 152:556–62.
Sanger F, Nicklen S, Coulson AR (1977)Proc Natl Acad Sci USA 74:5463–67.
Maniatis T, Fritsch EF, Sambrook J (1982)Molecular cloning: a laboratory manual. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory.
von Heijne G (1986)Nucleic Acids Res 11:4683–90.
Barondes SH (1988)TIBS 13:480–2.
Author information
Authors and Affiliations
Additional information
Note: The nucleotide sequences reported in this paper will appear in the Genbank™/EMBL Data library with the accession numbers L18894, L18895 and U07787.
Rights and permissions
About this article
Cite this article
van Damme, E.J.M., Balzarini, J., Smeets, K. et al. The monomeric and dimeric mannose-binding proteins from the Orchidaceae speciesListera ovata andEpipactis helleborine: sequence homologies and differences in biological activities. Glycoconjugate J 11, 321–332 (1994). https://doi.org/10.1007/BF00731205
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00731205