Abstract
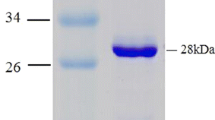
The gene encoding thermostable L-2-halo acid dehalogenase ofPseudomonas sp. YL was isolated, and its over-expression system was constructed. Gene library was prepared fromSau3AI fragments of total DNA fromPs. sp. YL, pUC118 as a vector andEscherichia coli JM109 as a host. The recombinant cells resistant to bromoacetate, a germicide, were isolated and shown to produce L-2-halo acid dehalogenase. Subsequently, subcloning was carried out with pKK223-3 as a vector, and the length of DNA inserted was reduced to 1.1 kbp. One of the subclones showed very high activity, and the amount of the dehalogenase produced corresponded to about 30% of the soluble protein. From 5 g (wet weight) of cells, 105 mg of dehalogenase was efficiently purified by heat treatment and DEAE-Toyopearl chromatography. This overexpression system provides a large amount of the thermostable enzyme to enable us to study the properties, structure and application of the enzyme.
Similar content being viewed by others
Abbreviations
- IPTG:
-
isopropyl β-D-thiogalactopyranoside
- KPB:
-
potassium phosphate buffer
- SDS:
-
sodium dodecyl sulfate
- X-gal:
-
5-bromo-4-chloro-3-indolyl-β-D-galactoside
References
Babbitt PC, Kenyon GL, Martin BM, Charest H, Sylvestre M, Scholten JD, Chang KH, Liang PH & Dunaway-Mariano D (1992) Ancestry of the 4-chlorobenzoate dehalogenase: analysis of amino acid sequence identities among families of acyl: adenyl ligases, enoyl-CoA hydratases/isomerases, and acyl-CoA thioesterases. Biochemistry 31: 5594–5604
Iwasaki I, Utsumi S, Hagino K & Ozawa T (1956) A new spectrophotometric method for the determination of small amounts of chloride using the mercuric thiocyanate method. Bull. Chem. Soc. Jpn. 29: 860–864
Janssen DB, Pries F, van der Ploeg J, Kazemier B, Terpstra P & Witholt B (1989) Cloning of 1,2-dichloroethane degradation genes ofXanthobacter autotrophicus GJ10 and expression and sequencing of the dhlA gene. J. Bacteriol. 171: 6791–6799
Jones DH, Barth PT, Byrom D & Thomas CM (1992) Nucleotide sequence of the structural gene encoding a 2-haloalkanoic acid dehalogenase ofPseudomonas putida strain AJ1 and purification of the encoded protein. J. Gen. Microbiol. 138: 675–683
Liu J, Kurihara T, Hasan AKMQ, Nardi-Dei V, Koshikawa H, Esaki N & Soka K (1994) Purification and characterization of thermostable and nonthermostable 2-haloacid dehalogenases with different stereospecificities fromPseudomas sp. strain YL. Appl. Environ. Microbiol. 60:2389–2393
Motosugi K, Esaki N & Soda K (1982a) Purification and properties of 2-halo acid dehalogenase fromPseudomonas putida. Agric. Biol. Chem. 46: 837–838
—— (1982b) Purification and properties of a new enzyme, DL-haloacid dehalogenase, fromPseudomonas sp. J. Bacteriol. 150: 522–527
Orser CS, Dutton J, Lange C, Jablonski P, Xun L & Hargis M (1993) Characterization of aFlavobacterium glutathione S-transferase gene involved reductive dechlorination. J. Bacteriol. 175: 2640–2644
Saito H & Miura K (1963) Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim. Biophys. Acta. 72: 619–629
Sambrook J, Fritsch EF & Maniatis T (1989) ‘Molecular Cloning: A Laboratory Manual; Second Edition,’ Cold Spring Harbor Laboratory Press
Shneider B, Muller R, Frank R & Lingens F (1991) Complete nucleotide sequences and comparison of the structural genes of two 2-haloalkanoic acid dehalogenases fromPseudomonas sp. strain CBS3. J. Bacteriol. 173: 1530–1535
Smith JM, Harrison K & Colby J (1990) Purification and characterization of D-2-haloacid dehalogenase fromPseudomonas putida strain AJ1/23. J. Gen. Microbiol. 136: 881–886
Thomas AW, Slater JH & Weightman AJ (1992) The dehalogenase gene dehI fromPseudomonas putida PP3 is carried on an unusual mobile genetic element designated DEH. J. Bacteriol. 174: 1932–1940
Van der Ploeg J, van Hall G & Janssen DB (1991) Characterization of the haloacid dehalogenase fromXanthobacter autotrophicus GJ10 and sequencing of the dhIB gene. J. Bacteriol. 173: 7925–7933
Verschueren KHG, Seljee F, Rozeboom HJ, Kalk KH & Dijkstra BW (1993) Crystallographic analysis of the catalytic mechanism of haloalkane dehalogenase. Nature 363: 693–698
Weightman AJ, Weightman AL & Slater JH (1982) Stereospecificity of 2-monochloropropionate dehalogenation by the two dehalogenases ofPseudomonas putida PP3: evidence for two different dehalogenation mechanisms. J. Gen. Microbiol. 128: 1755–1762
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, JQ., Kurihara, T., Nardi-Dei, V. et al. Overexpression and feasible purification of thermostable L-2-halo acid dehalogenase ofPseudomonas sp. YL. Biodegradation 6, 223–227 (1995). https://doi.org/10.1007/BF00700461
Issue Date:
DOI: https://doi.org/10.1007/BF00700461