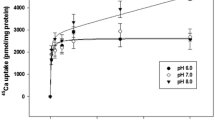
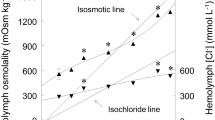
Summary
Glycera dibranchiata red coelomocytes regulate cell volume during hypoosmotic stress by reducing the intracellular concentration of certain osmotically active solutes, principally free amino acids (FAA) and K+. The volume regulatory mechanisms found in other species, especially the bivalve molluscs, are sensitive to changes in external divalent cation concentrations and the depletion of intracellular ATP. TheGlycera coelomocyte volume regulatory mechanism also requires divalent cations, but the requirement is not specific; either Ca2+ or Mg2+ will suffice. Deletion of both Ca2+ and Mg2+ from the external medium caused leakage of both FAA and K+ from the coelomocytes. The presence of the cellular ATP synthesis inhibitor DNP potentiated the volume regulatory response. The enhanced volume regulation was caused by a potentiated decrease of intracellular K+ content while the salinity induced FAA efflux was unaffected. Incubation of the coelomocytes with ouabain did not affect volume regulation, indicating that control of intracellular K+ during hypoosmotic stress does not depend on the Na+−K+-ATPase. Thus, the volume regulatory mechanisms in theGlycera coelomocytes utilize two types of solute, FAA and K+, and separate permeability mechanisms that interact to control the solute contents during hypoosmotic stress.
Similar content being viewed by others
Abbreviations
- FAA :
-
free amino acid
- DNP :
-
2,4-dinitrophenol
- ASW :
-
artificial seawater;mosm milliosmolal
- MOPS :
-
morpholinopropanesulfonic acid
- MCV :
-
mean cell volume
- MCV :
-
mean-mean cell volume
- CCCP :
-
carbonyl cyanide m-chlorophenylhydrazone
- RVD :
-
regulatory volume decrease
References
Amende LM, Pierce SK (1980a) Cellular volume regulation in salinity stressed molluscs: The response ofNoetia ponderosa (Arcidae) red blood cells to osmotic variation. J Comp Physiol 138:283–289
Amende LM, Pierce SK (1980b) Free amino acid mediated volume regulation of isolatedNoetia ponderosa red cells: Control by Ca2+ and ATP. J Comp Physiol 138:291–298
Amende LM, Pierce SK (1980c) Structural changes ofNoetia ponderosa red blood cell membranes during cell volume regulation in reduced salinities: A freeze fracture study. J Comp Physiol 138:299–306
Burton RF (1968) Cell potassium and the significance of osmolarity in vertebrates. Comp Biochem Physiol 27:763–773
Burton RF (1973) The significance of ionic concentrations in the internal media of animals. Biol Rev 48:195–231
Cala PM (1977a) Volume regulation by flounder red cells: The role of the membrane potential. J Exp Zool 199:339–344
Cala PM (1977b) Volume regulation by flounder red blood cells in anisotonic media. J Gen Physiol 69:537–552
Costa CJ, Pierce SK, Warren MK (1980) The intracellular mechanisms of salinity tolerance in polychaetes: Volume regulation by isolatedGlycera dibranchiata red coelomocytes. Biol Bull 159:626–638
Freel RW, Medler SG, Clark ME (1973) Solute adjustments in the coelomic fluid and muscle fibers of a euryhaline polychaete,Neanthes succinea adapted to various salinities. Biol Bull 144:289–303
Hauser HB, Levine BA, Williams RJP (1976) Interactions of ions with membranes. Trends Biochem Sci 1:278–281
Hendil K, Hoffmann EK (1974) Cell volume regulation in Ehrlich ascites tumor cells. J Cell Physiol 84:115–126
Heytler PG, Pritchard WW (1962) A new class of uncoupling agents — carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun 7:272–275
Hoffmann EK (1980) Cell volume regulation in mammalian cells. In: Gilles R (ed) Animals and environmental fitness. Pergamon Press, Oxford, pp 43–59
Kevers C, Pequeux A, Gilles R (1979a) Effects of an hypoosmotic shock on Na+, K+, and Cl− levels in isolated axons ofCarcinus maenas. J Comp Physiol 129:365–371
Kevers C, Pequeux A, Gilles R (1979b) Effects of hypo- and hyper-osmotic shocks on the volume and ion content ofCarcinus maenas isolated axons. Comp Biochem Physiol 64A:427–431
Kevers C, Pequeux A, Gilles R (1981) Role of K+ in the cell volume regulation response of isolated axons ofCarcinus maenas submitted to hypo-osmotic conditions. Mol Physiol 1:13–22
Kessler RJ, Vande Zande H, Tyson CA, Blondin GA, Fairfield J, Glasser P, Green DE (1977) Uncouplers and the molecular mechanism of uncoupling in mitochondria. Proc Natl Acad Sci USA 74:2241–2245
Kregenow FW (1971) The response of duck erythrocytes to nonhemolytic hypotonic media. J Gen Physiol 58:372–412
Laclette JP, Montal M (1977) Interaction of calcium with negative lipids in planar bilayer membranes. Influence of the solvent. Biophys J 19:199–202
Lauf PK (1982) Evidence for chloride dependent potassium and water transport induced by hypoosmotic stress. J Comp Physiol 146:9–16
Machin J (1975) Osmotic responses of the bloodwormGlycera dibranchiata (Ehlers): A graphical approach to the analysis of weight regulation. Comp Biochem Physiol 52A:49–54
Manery JF (1966) Effects of Ca++ ions on membranes. Fed Proc 25:1804–1810
Meech RW (1976) Intracellular calcium and the control of membrane permeability. In: Duncan CJ (ed) Calcium in biological systems. Cambridge University Press, Cambridge, pp 161–191
Parker JC, Gitelman HJ, Glosson PS, Leonard DL (1975) Role of calcium in volume regulation by dog red blood cells. J Gen Physiol 65:84–96
Parker JC, Castranova V, Goldinger JM (1977) Dog red blood cells: Na+ and K+ diffusion potentials with extracellular ATP. J Gen Physiol 69:417–430
Pierce SK (1982) Invertebrate cell volume control mechanisms: A corrdinated use of intracellular amino acids and inorganic ions as osmotic solute. Biol Bull 163:405–419
Pierce SK, Jr, Greenberg MJ (1972) The nature of cellular volume regulation in marine bivalves. J Exp Biol 57:681–692
Pierce SK, Jr, Greenberg MJ (1973) The initiation and control of free amino acid regulation of cell volume in salinity stressed marine bivalves. J Exp Biol 59:435–446
Pierce SK, Jr, Greenberg MJ (1976) Hypoosmotic cell volume regulation in marine bivalves: The effects of membrane potential change and metabolic inhibition. Physiol Zool 49:417–424
Rasmussen H (1975) Ions as second messengers. In: Weismann G, Claiborne R (eds) Cell membranes, biochemistry, cell biology, and pathology. H P Publishing, New York, pp 203–212
Rorive G, Gilles R (1979) Intracellular inorganic effectors. In: Gilles R (ed) Mechanisms of osmoregulation in animals. Wiley, New York, pp 83–110
Rorive G, Kleinzeller A (1972) The effect of ATP and Ca++ on the cell volume in isolated kidney tubules. Biochim Biophys Acta 274:226–239
Schmidt-Nielsen B (1975) Comparative physiology of cellular ion and volume regulation. J Exp Zool 194:207–220
Schulz I, Heil K (1979) Ca2+ control of electrolyte permeability in plasma membrane vesicles from cat pancreas. J Membr Biol 46:41–70
Sokal RR, Rohlf J (1969) Introduction to biostatistics. Freeman, San Francisco, California
Steel RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw-Hill, New York
Warren MK, Pierce SK (1982) Two cell volume regulatory systems in theLimulus myocardium: An interaction of ions and quarternary ammonium compounds. Biol Bull 163: 504–516
Watts JA, Pierce SK (1978a) Characterization of the divalent cation activated adenosine triphosphatase from the membrane of the cardiac cell ofModiolus demissus. J Exp Zool 204:43–48
Watts JA, Pierce SK (1978b) A correlation between the activity of the divalent cation activated adenosine triphosphatase in the cell membrane and low salinity tolerance of the ribbed mussel,Modiolus demissus demissus. J Exp Zool 204: 49–56
Wilkens LA (1972) Electrophysiological studies on the heart of the bivalve mollusc,Modiolus demissus. I. Ionic basis of the membrane potential. J Exp Biol 56:273–391
Wins P (1969) The interaction of red cell membrane ATPase with calcium. Arch Int Physiol Biochim 77:245–250
Wins P, Schoffeniels E (1966) Studies on red-cell ghost ATP systems; properties of a (Mg2++Ca2+)-dependent ATPase. Biochim Biophys Acta 120:332–340
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Costa, C.J., Pierce, S.K. Volume regulation in the red coelomocytes ofGlycera dibranchiata: An interaction of amino acid and K+ effluxes. J Comp Physiol B 151, 133–144 (1983). https://doi.org/10.1007/BF00689911
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00689911