Summary
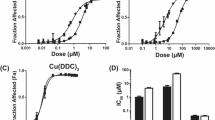
We have previously shown that the toxicity of valinomycin (VM), a membrane-active agent with antineo-plastic activity, can be dramatically reduced with no loss of the antitumor efficacy of the drug by incorporating it into liposomes. In the present study, we investigated the interaction betweencis-diamminedichloroplatinum(II) (CDDP) and VM in terms of in vitro cytotoxicity to human ovarian tumor cells. Using the MTT assay and analyzing the data using the median-effect principle, we showed that synergistic cytotoxic interactions exist between CDDP and VM in their liposomal form. The degree of cytotoxic synergism was influenced by the duration of drug exposure and the dose ratio. The cellular accumulation of platinum by ovarian cells at 37°C was slightly higher after exposure to VM as compared with controls; however, it is not clear that this accounts for the cytotoxic synergism. These results suggest that the combination of liposomal VM and CDDP may have merit as a form of localized drug delivery for the treatment of ovarian cancer disseminated within the peritoneal space.
Similar content being viewed by others
Abbreviations
- CDDP:
-
cisplatin,cis-diamminedichloroplatinum(II)
- VM:
-
valinomycin
- MLV-VM:
-
liposomal valinomycin
- MTT:
-
3-(4,5-dimethyl thiazol-2-yl)-2,5 diphenyl-tetrazolium bromide (thiazolyl blue)
- alpha-MEM:
-
alpha minimal essential medium
- CHO:
-
Chinese hamster ovary
- IC50 :
-
concentration causing 50% inhibition of cell growth
- IC10 :
-
concentration causing 10% inhibition of cell growth
- SF:
-
surviving fraction
- fa:
-
fraction affected
- CI:
-
combination index
References
Alley MC, Scudiero DA, Monks A, Hunsey ML, Czerwinski MJ, Pine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR (1989) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48: 589
Berenbaum MC (1978) A method for testing for synergy with a number of agents. J Infect Dis 137 (2): 122
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248
Burns CP (1988) Membranes and cancer chemotherapy. Cancer Invest 6 (4), 439
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB (1987) Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47: 936
Carter SK (1984) Cisplatin-past, present and future. In: Haker MP, Douple EB, Krakoff IH (eds) Platinum coordination complexes in cancer chemotherapy. Martinus Nijhoff, Boston, pp 295
Chabot GG, Valeriote FA (1986) Modification of cell sensitivity to anticancer agents by polyenes. In: Valeriole FA, Baker LH (eds) Biochemical modulation of anitcancer agents: experimental and clinical approaches. Martinus Nijhoff, Boston, p 295
Chou J, Chou T-C (1987) Dose-effect analysis with microcomputers. Biosoft, Cambridge
Chou T-C, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22: 27
Chou T-C, Talalay P (1987) Application of the median-effect principle for the assessment of low-dose risk of carcinogens and for the quantitation of synergism and antagonism of chemotherapeutic agents. In: Harrap KR, Connors TA (eds) New avenues in developmental cancer chemotherapy. Academic Press, New York, p 37
Daoud SS, Juliano RL (1986) Enhancement of the therapeutic index of valinomycin by incorporation in liposomes. Proc Am Assoc Cancer Res 27: 409
Daoud SS, Juliano RL (1986) Reduced toxicity and enhanced antitumor effects in mice of the ionophoric drug valinomycin when incorporated in liposomes. Cancer Res 46: 5518
Daoud SS, Juliano RL (1989) In vitro effect of liposome-incorporated valinomycin on growth and macromolecular synthesis of normal and ras-transformed 3T3 cells. Cancer Chemother Pharmacol 23: 151
Daoud SS, Juliano RL (1989) Modulation of doxorubicin resistance by valinomycin (NSC-122023) and liposomal valinomycin in Chinese hamster ovary cells. Cancer Res 49: 2661
Daoud SS, Hume LE, Juliano RL (1989) Liposomes in cancer therapy. Adv Drug Rev 3: 405
Durand RE, Goldie JH (1987) Interaction of etoposide and cisplatin in an in vitro tumor model. Cancer Treat Rep 72: 673
Eastman A (1984) Characterization of the interaction of cis-diamminedichloroplatinum(II) with DNA. In: Haker MP, Douple EB, Krakoff IH (eds) Platinum coordination complexes in cancer chemotherapy. Martinus Nijhoff, Boston, p 56
Einhorn LH, Donohue J (1977) cis-diamminedichloroplatinum(II), vinblastine and bleomycin combination therapy in disseminated testicular cancer. Ann Intern Med 87: 293
Erickson LC, Zwelling LA, Ducore JM, Sharkey NA, Kohn KW (1981) Differential cytotoxicity and DNA crosslinking in normal and transformed human fibrobasts treated with cis-diamminedichloroplatinum(II) in vitro. Cancer Res 41: 2791
Forde NH, Daoud SS (1990) Cisplatinum and liposomal valinomycin in ovarian cancer: cytoxicity synergism in vitro. Proc Am Assoc Cancer Res 31: 2298
Friedman SJ, Skehan P (1984) Cell membranes: targets of selective antitumor chemotherapy. In: Prased SS (ed) Novel approaches to cancer chemotherapy. Academic Press, New York, p 329
Grinstein S, Cohen S, Rothstein A (1984) Cytoplasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport. J Gen Physiol 83: 341
Grinstein S, Rotin D, Mason MJ (1989) Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta 988: 73
Hoelzl-Wallach DF, Mikkelsen RB, Kwock L (1981) Plasma membrane as targets and mediators. In: Sartorelli AC, Lazo JS, Bertino JR (eds) Tumor chemotherapeutic agents. Academic Press, New York, p 433
Jackson MJ (1986) Weak electrolyte transport across biological membranes: general principles. In: Andreoli TE, Hoffman JF, Fanestil DD, Schultz SG (eds) Physiology of membrane disorders. Plenum Press, New York London, p 235
Jacobs C (1980) The role of cisplatin in the treatment of recurrent head and neck cancer. In: Prestayko AW, Crooke ST, Carter SK (eds) Cisplatin F current status and new developments. Academic Press, New York, p 423
Kikuchi Y, Oomori K, Kizawa I, Hirata J, Kita T, Miyauchi M (1987) Enhancement of antineoplastic effects of cisplatin by calmodulin antagonists in nude mice bearing human ovarian carcinoma. Cancer Res 47: 6459
Kitagawa S, Awai M, Kametani F (1987) Relationship of the effects of nigericin on the aggregation and cytoplasmic pH of bovine platelets in the presence of different cations. Biochim Biophys Acta 930: 48
Kleuser B, Rieter H, Adam G (1985) Selective effects by valinomycin on cytotoxicity and cell cycle arrest of transformed versus non-transformed rodent fibroblasts in vitro. Cancer Res 45: 3022
Krakoff IH (1979) Nephrotoxicity of cis-dichlorodiammineplatinum(II). Cancer Treat Rep 63: 1523
Loehrer PJ, Einhorn LH (1984) Diagnosis and treatment. Drugs five years later: cisplatin. Ann Intern Med 100: 704
Markman M (1985) Melphalan and cytarabine administered intraperitoneally as single agents and combination intraperitoneal chemotherapy with cisplatin and cytarabine. Semin Oncol 12 (3): 33
Mauldin SK, Gibbons G, Wyrick SD, Chaney SG (1988) Intracellular biotransformation of platinum with the 1,2-diaminocyclohexane carrier ligand in the L1210 cell line. Cancer Res 48: 5136
Medias NE, Harrington JT (1978) Platinum nephrotoxicity. Am J Med 65: 307
Moolenar WH (1986) Effects of growth factors on intracellular pH regulation. Annu Rev Physiol 48: 363
Onoda JM, Nelson KK, Taylor JD, Honn KV (1989) In vivo characterization of combination antitumor chemotherapy with calcium channel blockers and cis-diamminedichloroplatinum(II). Cancer Res 49: 2844
Ozols RF, Young RC (1984) Chemotherapy of ovarian cancer. Semin Oncol 11: 251
Park J-G, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, Gazdar AF (1987) Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based assay. Cancer Res 47: 5875
Plumb JA, Milroy R, Kaye SB (1989) Effects of pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res 49: 4435
Pouyssegur J, Sardet C, Franchi A, L'Allemain G, Paris S (1984) A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci USA 81: 4833
Randoloph VL, Vallejo A, Spiro RH, Shah J, Strong E, HuVos A, Wittes R (1978) Combination therapy of advanced head and neck cancer. Cancer 41: 460
Roberts JJ, Thomson AJ (1979) The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol 22: 71
Rossof AH, Slayton RE, Perlia CP (1972) Preliminary clinical experience with cis-diamminedichloroplatinum(II) (NSC-119 875, CACP). Cancer 30: 1415
Rotin D, Wan P, Grinstein S, Tannock I (1987) Cytotoxicity of compounds that interfere with the regulation of intracellular pH: a potential new class of anticancer drugs. Cancer Res 47: 1497
Sorenson CM, Eastman A (1988) Mechanism of cis-diamminedi-chloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res 48: 4484
Swinnen LJ, Barnes DM, Fisher SG, Albain KS, Fisher RI, Eickerson LC (1989) 1-β-d-Arabinofuranosylcytosine and hydroxyurea production of cytotoxic synergy with cis-diamminedichloroplatinum(II) and modification of platinum-induced DNA interstrand cross-linking. Cancer Res 49: 1383
Tritton TR, Hickman JA (1985) Cell membranes as a chemotherapeutic target. In: Experimental and Clinical Progress in Cancer Chemotherapy. Muggia FM (ed) Martinus Nijoff, Boston, pp 81–131
Tritton TR, Grace Y, Ehrlich YH (1985) Mechanisms of membrane-mediated cytotoxicity by Adriamycin. In: Chandra P (ed) New experimental modalities in the control of neoplasia. Plenum Press, New York London, p 195
Tycko B, Maxfield FR (1982) Rapid acidification of endocytic vesicles containing α2-macroglobulin. Cell 28: 643
Von Hoff DD, Schilsky R, Reichert CM, Reddick RL, Rozencweig M, Young RC, Muggia EM (1979) Toxic effects of cis-dichlorodiammineplatinum in man. Cancer Treat Rep 63: 1527
Zwelling LA, Khon RW (1980) Effects of cisplatin on DNA and the possible relationships to cytotoxicity and mutagenicity in mammalian cells. In: Prestayko AW, Crooke ST, Carter SK (eds) Cisplatin F current status and new developments. Academic Press, New York, p 21
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Daoud, S.S., Forde, N.H. Synergistic cytotoxic actions of cisplatin and liposomal valinomycin on human ovarian carcinoma cells. Cancer Chemother. Pharmacol. 28, 370–376 (1991). https://doi.org/10.1007/BF00685692
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685692