Abstract
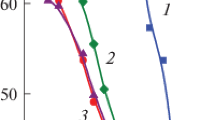
Carboxylated polystyrene latex particles were prepared by emulsifier-free emulsion polymerization of styrene using an azoinitiator (ACPA), which provides carboxyl end groups on the latex surface. Two latexes were characterized using TEM, PCS, conductimetric and potentiometric titrations, and electrophoretic mobility. To determine the hydrophobic or hydrophilic character of these latexes, the maximum adsorption of a nonionic surfactant (Triton X-100) was also studied and compared with other type of latexes. The electrophoretic mobility of these functionalized model colloids was studied in the presence of various types of inorganic electrolytes. The μ e curves of these latexes exhibit a smooth maximum at an electrolyte concentration of around 10−3 and 5·10−3 M for 1∶1, 2∶1 and 1∶2 electrolytes. When a 3∶1 electrolyte (LaCl3) was used, the electrophoretic mobility changed to positive values at high concentration due to the specific adsorption of lanthanum species. In general, the surface characteristics of these carboxylated latexes are very different in comparison to other latexes with the same functionality because the carboxyl groups are provided by the initiator, while in most of the cases these groups are provided by ionic comonomers (acrylic, methacrylic acids, etc.) used in the copolymerization with styrene.
Similar content being viewed by others
References
Tamai H, Fujii A, Suzawa T (1987) J Colloid Interface Sci 116:37
Rembaum A, Yen SPS, Cheong E, Wallace S, Molday RS, Gordon IL, Dreyer WJ (1976) Macromol 9:328
Tamai H, Hasegawa M, Suzawa T (1989) J Appl Polym Sci 38:403
Martin-Rodriguez A, Cabrerizo MA, Hidalgo-Alvarez R (1994) Colloids Surfaces A: Physicochem Eng Aspects 92:113
Bastos-González D, Ortega-Vinuesa JL, De las Nieves FJ, Hidalgo-Alvarez R (1995) J Colloid Interface Sci 176:232
Guthrie WH (1985) Ph D Dissertation, Lehigh University, USA
Shubin VA, Hunter RJ, O'Brien (1993) J Colloid Interface Sci 176:232
Martín-Rodríguez A (1993) Ph D Dissertation, University of Granada, Spain
Bastos D, de las Nieves FJ (1993) Colloid Polym Sci 271:860
de las Nieves FJ, Daniels ES, El-Aasser MS (1991) Colloids Surfaces 60:107
Tamai H, Niino K, Suzawa T (1989) J Colloid Interface Sci 131:1
Zukoski CF, Saville DA (1985) J Colloid Interface Sci 107:322
Van der Put AG, Bijsterbosch BH (1983) J Colloid Interface Sci 92:499
Prescott JH, Shiau S, Rowell RL (1993) Langmuir 9:2071
Marlow BJ, Rowell RL (1991) Langmuir 7:2970
Seebergh JE, Berg JC (1995) Colloids Surfaces A: Physicochem Eng Aspects 100:139
Gittings MR, Saville DA (1995) Langmuir 11:798
Elimelech M, O'Melia CR (1990) Colloids Surfaces 44:165
Van Streun KH, Belt WJ, Piet P, German AL (1991) Eur Polym J 27:931
Goodwin JW, Hearn J, Ho CC, Ottewill RH (1974) Z Z Polym 252:464
Baran AA, Dukhina LM, Soboleva NM, Chechik OS (1981) Kolloid Z 43:211
Hidalgo-Alvarez R (1991) Adv Colloid Interface Sci 34:217
Moleón-Baca JA, Rubio-Hernández FJ, de las Nieves, Hidalgo-Alvarez R (1991) J Non-Equil Thermodyn 16:187
Hunter RJ (1981) In: Ottewill RH, Rowel RL (eds) Zeta-Potential in Colloid Science. Academic Press, New York
Midmore BR, Hunter RJ (1988) J Colloid Interface Sci 122:521
Chow RS, Takamura K (1988) J Colloid Interface Sci 125:226
Shirahama H, Suzawa T (1984) Polymer J 16:795
Ottewill RH, Shaw JN (1968) J Colloid Interface Sci 26:110
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bastos, D., de las Nieves, F.J. Effect of electrolyte type on the electrokinetic behavior of carboxylated polystyrene model colloids. Colloid Polym Sci 274, 1081–1088 (1996). https://doi.org/10.1007/BF00658373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00658373