Abstract
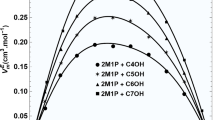
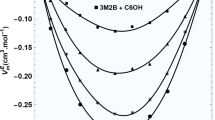
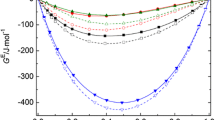
The pressure dependence of the excess enthalpy HE, dHE/dP, has been calculated from experimental excess volumes VE and dVE/dT using dHE/dP=VE−TdVE/dT. dHE/dP at zero pressure are reported at 25°C and equimolar concentration for the mixtures: cyclohexane with the series of normal alkanes (n-C n , where n=6,8,10,12,14 and 16) and with the series of highly branched alkanes (br-C n , where n=6,8,12 and 16), benzene, toluene and p-xylene +n-C n and 1-chloronaphthalene +n-C n and br-C n . Experimental and Flory theory dHE/dP values are in good agreement for the whole cyclohexane +br-C n series. For the n-C n series, dHE/dP becomes increasingly positive deviating from the Flory predictions. This discrepancy is due to the presence of short-range orientational order in the higher n-C n pure liquids which makes dH/dP more negative and which, upon mixing, is destroyed producing a positive contribution to dHE/dP not accounted for by the theory. The discrepancy between theoretical and experimental dHE/dP is large for benzene, but progressively smaller for toluene, p-xylene and 1-chloronaphthalene. These results are consistent with creation of order between the aromatic plate-like molecule and the long n-C n in solution. For 1-chloronaphthalene +n-C n , this order creation process produces a negative contribution to dHE/dP which balances the positive order-destruction contribution originated by the rupture, upon mixing, of short-range orientational order in pure n-C n .
Similar content being viewed by others
References
M. Costas and D. Patterson,Thermochim. Acta 120, 161 (1987);
E. Wilhelm, ibid94, 47 (1985)
H. Wagner and R. L. Lichtenthaler ibid,94, 67 (1985)
S. N. Bhattacharyya, M. Costas, D. Patterson and V. H. Tra,Fluid Phase Equil. 20, 27 (1985).
S. N. Bhattacharyya and D. Patterson,J. Chem. Soc. Faraday Trans. I 81, 375 (1985)
M. Costas, S. N. Bhattacharyya and D. Patterson, ibid81, 387 (1985)
S. N. Bhattacharyya and D. Patterson,J. Phys. Chem. 83, 2979 (1979)
E. Aicart, G. Tardajos and M. Diaz-Peña,J. Chem. Eng. Data 25, 140 (1980)
G. Tardajos and M. Diaz-Peñ, ibid26, 22 (1981)
E. Aicart, G. Tardajos and M. Diaz-Peña,J. Chem. Thermodyn. 13, 783 (1981)
E. Aicart, C. Menduiña, R. L. Arenosa and G. Tardajos,J. Solution Chem. 13, 443 (1984).
J. P. E. Grolier, A. Faradjzadeh and H. V., Kehiaian,Thermochim. Acta 53, 157 (1982)
M. Diaz-Peña, G. Tardajos, C. Menduiña and R. L. Arenosa,J. Chem. Thermodyn. 11, 67 (1979)
M. Diaz-Peña, G. Tardajos, C. Menduiña and R. L. Arenosa, ibid11, 951 (1979)
E. Aicart, C. Menduiña, R. L. Arenosa and G. Tardajos,J. Solution Chem. 12, 703 (1983)
G. Tardajos, E. Aicart and M. Diaz-Peña,Fluid Phase Equil. 20, 87 (1985).
G. Tardajos, E. Aicart, M. Costas and D. Patterson,J. Chem. Soc. Faraday Trans I 82, 2977 (1986)
M. Costas and D. Patterson,Int. Data Ser. A. 3, 212 (1985).
J. Garbajosa, G. Tardajos, E. Aicart and M. Diaz-Peña,J. Chem. Thermodyn. 14, 671 (1982).
I. Gamboa, E. Aicart, M. Diaz-Peña and G. Tardajos, ibid18, 885 (1986).
C. C. Gonzalez, M. Cáceres-Alonso and J. Nuñez,J. Solution Chem. 15, 33 (1986).
E. Picquenard, H. V. Kehiaian, L. Abello and G. Pannetier,Bull. Chim. Fr. 120, (1970).
M. Costas, H. T. Van, D. Patterson, M., Cáceres-Alonso, G. Tardajos and E. Aicart,J. Chem. Soc. Faraday Trans. I 84, 1603 (1988).
J. P. E. Grolier, A. Inglese, A. H. Roux and E. Wilhelm,Ber. Bunsenges. Phys. Chem. 85, 768 (1981).
P. J. Flory,J. Amer. Chem. Soc. 87, 1833 (1965)
A. Abe and P. J. Flory, ibid87, 1838 (1965).
J. J. Christensen, R. M. Izatt, D. J. Eatough and D. L. Hansen,J. Chem. Thermodyn. 10, 25 (1978).
J. R. Goates, J. B. Ott and J. F. Moellmer,J. Chem. Thermodyn. 9, 249 (1977).
A. Heintz and R. N. Lichtenthaler,Ber. Bunsenges. Phys. Chem. 84, 727 (1980).
A. Heintz and R. N. Lichtenthaler, ibid84, 890 (1980).
H. T. Van and D. Patterson,J. Solution Chem. 11, 793 (1982).
V. T. Lam, P. Picker, D. Patterson and P. Tancrede,J. Chem. Soc. Faraday Trans II 70, 1465 (1974).
M. Costas and D. Patterson,J. Solution Chem. 11, 807 (1982).
P. de St Romain and D. Patterson,J. Solution Chem. 11, 119 (1982)
P. de St Romain, H. T. Van and D. Patterson,J. Chem. Soc. Faraday Trans I 75, 1700 (1979).
E. Wilhelm, A. Inglese, J. P. E. Grolier and H. V. Kehiaian,Ber. Bunsenges. Phys. Chem. 82, 384 (1978)
A. Inglese, J. P. E. Grolier and E. Wilhelm,Fluid Phase Equil. 15, 287 (1984)
J. P. E. Grolier, A. Inglese, A. H. Roux and E. Wilhelm, “Chemical and Engineering Thermodynamics”, S. A. Newman, ed.,Proceedings of the World Congress of Chemical Engineering, (Ann Arbor Science. Montreal, 1981), Chap. 40, p. 483.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aicart, E., Tardajos, G. & Costas, M. The effect of pressure on order destruction and order creation in linear or branched alkane mixtures. J Solution Chem 18, 369–377 (1989). https://doi.org/10.1007/BF00656774
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00656774