Abstract
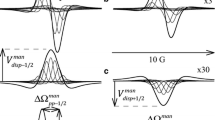
Electron spin exchange rate constants and hyperfine coupling constants have been measured for two nitroxide spin probes in a number of isoviscous mixed solvents. Collision rate constants, normalized to 1 cP, are lower for solvents in which the major component is water. Further anomalies in the coupling constant for the systemtert-butanol-water are explained in terms of the marked concentration fluctuations known to occur in this solvent mixture. Substitution of a hydrophobic spin probe by one containing an −OH group leads to lowering of the spin exchange rate, possibly due to solvation of the probe.
Similar content being viewed by others
References
S. Ablett, M. D. Barratt, and F. Franks,J. Solution Chem. 4, 797 (1975).
E. v. Goldammer and H. G. Hertz,J. Phys. Chem. 74, 3734 (1970).
G. Ebert and J. Wendorff,Ber. Bunsenges. Phys. Chem. 74, 1071 (1970).
T. A. Miller, R. N. Adams, and P. M. Richards,J. Chem. Phys. 44, 4022 (1966).
A. Carrington and A. D. McLachlan,Introduction to Magnetic Resonance (Harper and Row, London, 1967).
E. G. Rozantzev and M. B. Neiman,Tetrahedron 20, 131 (1964).
R. A. Robinson and R. H. Stokes,J. Phys. Chem. 65, 1954 (1961).
F. Franks and D. S. Reid, inWater—A Comprehensive Treatise, Vol. 2, F. Franks, ed. (Plenum Press, New York, 1973), p. 323.
F. Franks and M. D. Pedley, unpublished results.
G. Stout and J. B. F. N. Engberts,J. Org. Chem. 39, 3800 (1974).
J. Gendell, J. H. Freed, and G. K. Fraenkel,J. Chem. Phys. 37, 2832 (1962).
H. D. Bale, R. E. Shepler, and D. K. Sorgen,Phys. Chem. Liq. 1, 181 (1968).
F. Franks, inWater—A Comprehensive Treatise, Vol. 4, F. Franks, ed. (Plenum Press, New York, 1975), p. 1.
Y. Y. Lim, E. A. Smith, and M. C. R. Symons,J. Chem. Soc. Faraday Trans. 1 72, 2876 (1976).
C. Jolicoeur and H. L. Friedman,J. Solution Chem. 3, 15 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barratt, M.D., Franks, F. & Robinson, P.N. Electron spin exchange in isoviscous solvent mixtures. J Solution Chem 6, 625–631 (1977). https://doi.org/10.1007/BF00655375
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00655375