Abstract
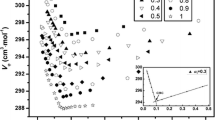
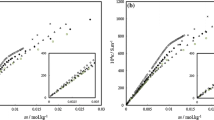
Density measurements of water-dodecyltrimethylammonium bromide (DTAB)-alcohol ternary systems as a function of alcohol and surfactant concentrations were carried out at 25°C. The alcohols were propanol (PrOH), 2-propanol (2-PrOH) and hexanol (HexOH). The apparent molar volume Vϕ,R of alcohols have been calculated and the standard (infinite dilution) partial molar volumes of alcohols V R at each surfactant concentration were obtained by means of a least squares fit of Vϕ,R vs. the alcohol concentration. The V R vs. surfactant concentration curves have been rationalized in terms of the partial molar volume of alcohol in the aqueous V f and the micellar V b phases and the distribution constant of alcohol between the aqueous and the micellar phases K. The V b values for PrOH and HexOH together with those of butanol and pentanol previously reported satisfy the additivity rule giving a methylene group contribution of 16.7 cm3-mol−1 which is identical to that reported in the literature from the study of pure liquid alcohols. No difference between V b for PrOH and 2-PrOH has been found. From density data of water-alcohol and water-surfactant binary systems and of water-surfactant-alcohol ternary system, the apparent molar volume of the surfactant in the water-alcohol mixed solvent Vϕ,S have been calculated as a function of the surfactant concentration and of the mixed solvent composition. The effect of the alkyl chain length of the alcohols and the effect of isomerization of the alcohols on the Vϕ,S vs. surfactant concentration trends have been analyzed.
Similar content being viewed by others
References
Surfactants Solutions: New Methods of Investigation, R. Zana, ed., (M. Dekker Inc., NY, 1986).
Solubilization and Microemulsions, K. L. Mittal, ed., Micellization, (Plenum Press, NY, 1977).
Solution Chemistry of Surfactants, K. L. Mittal, ed., (Plenum Press, NY, 1979).
Micellization, Solution Behavior of Surfactants: Theoretical and Applied Aspects, K. L. Mittal and E. Fendler, eds., (Plenum Press, NY, 1982).
Surfactants in Solution, Vol. 1–3, K. L. Mittal and B. Lindman, eds., (Plenum Press, NY, 1984).
Surfactants in Solution, Vol. 4–6, K. L. Mittal and P. Bothorel, eds., (Plenum Press, NY, 1986).
H. Høiland, E. Ljosland, and S. Backlund,J. Coll. Interf. Sci. 101, 467 (1984).
E. Vikingstad,J. Coll. Interf. Sci. 72, 75 (1979).
J. E. Desnoyers, D. Hétu, and G. Perron,J. Solution Chem. 12, 427 (1983).
C. Treiner,J. Coll. Interf. Sci. 93, 33 (1983).
R. De Lisi and S. Milioto,J. Solution Chem. 17, 245 (1988).
P. Stilbs,J. Coll. Interf. Sci. 87, 385 (1982).
G. Roux-Desgranges, A. H. Roux, and A. Viallard,J. Chim. Phys. 82, 441 (1985).
M. Manabe, S. Kikuchi, S. Katayama, S. Tokunaga, and M. Koda,Bull. Chem. Soc. Jpn. 57, 2027 (1984).
R. De Lisi, A. Lizzio, S. Milioto, and V. Turco Liveri,J. Solution Chem. 15, 623 (1986).
R. De Lisi, V. Turco Liveri, M. Castagnolo, and A. Inglese,J. Solution Chem. 15, 23 (1986).
R. De Lisi, S. Milioto, and R. Triolo,J. Solution Chem. 17, 673 (1988).
R. De Lisi, S. Milioto, and R. E. Verrall,J. Solution Chem. (in press).
J. Lara, G. Perron, and J. E. Desnoyers,J. Phys. Chem. 85, 1600 (1981).
G. S. Kell,J. Chem. Ing. Data 12, 66 (1967).
Handbook of Chemistry and Physics, 67th Edn., (CRC Press, Boca Ratan, 1986).
J. P. Grolier, A. Inglese, A. H. Roux, and E. Wilhem,Ber. Bunsenges. Phys. Chem. 85, 768 (1981).
W. McMillan and J. Mayer,J. Phys. Chem. 13, 176 (1945).
C. Jolicoeur and G. Lacroix,Can. J. Chem. 54, 624 (1976).
H. Høiland and E. Vikingstad,Acta Chem. Scand. A30, 182 (1976).
R. De Lisi and V. Turco Liveri,Gazzetta Chim. Ital. 13, 371 (1983).
R. Zana, S. Yiv, C. Strazielle, and P. Lianos,J. Coll. Interf. Sci. 80, 208 (1981).
R. De Lisi and S. Milioto,J. Solution Chem. 16, 767 (1987).
R. De Lisi, S. Milioto, and V. Turco Liveri,J. Coll. Interf. Sci. 117, 64 (1987).
S. Milioto and R. De Lisi,J. Coll. Interf. Sci. 123, 92 (1988).
H. Høiland,J. Solution Chem. 5, 773 (1976).
R. De Lisi, C. Genova, R. Testa, and V. Turco Liveri,J. Solution Chem. 13, 121 (1984).
A. H. Roux, D. Hétu, G. Perron, and J. E. Desnoyers,J. Solution Chem. 13, 1 (1984).
S. G. Bruun and A. Hvidt,Ber. Bunsenges. Phys. Chem. 81, 930 (1977).
C. Dethlefsen, P. G. Sørensen, and A. Hvidt,J. Solution Chem. 13, 191 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Lisi, R., Milioto, S., Castagnolo, M. et al. Standard partial molar volumes of alcohols in aqueous dodecyltrimethylammonium bromide solutions. J Solution Chem 19, 767–791 (1990). https://doi.org/10.1007/BF00647103
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00647103