Abstract
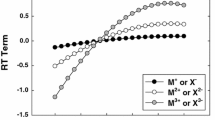
A radioactive tracer method has been used to determine the trnasference numbers of eight major ions in seawater of salinity 38.4 g-kg−1 at 25°C. Data are presented for Na+, K+, Ca2+, and Mg2+ cations and Cl−, Br−, SO 2− 4 , and HCO − 3 anions, which contribute to the overall electrical conductance by more than 99%. The results have been checked by electrophoretic migrations on esters of cellulose strips providing independent values of the ratios of ionic mobilities to be compared to previous estimations. Through the individual ionic contributions to the electrical conductance, a method for calculating seawater density is proposed; this method is based on a nine-constitutents seawater model in which relative ionic concentrations can deviate from that of standard seawater.
Similar content being viewed by others
References
A. Poisson and J. Chanu,AGARD Conf. Proc. No. 77, 24 (1970).
K. Park,Deep Sea Res. 11, 729 (1964).
M. Spiro, Transference Numbers, inPhysical Methods of Chemistry, A. Weissberger and B. W. Rossiter, eds., Part IV (Interscience, New York, 1971).
M. Périé, J. Périé, and M. Chemla,Electrochim. Acta 19, 753 (1974).
M. Périé, J. Périé, and M. Chemla,Electrochim. Acta 21, 739 (1976).
A. Poisson and J. Chanu,Limnol. Oceanogr.21, 853 (1976).
Z. Kniewald and Z. Pučar,Trans. Faraday Soc. 72, 987 (1976).
D. J. Currie and A. R. Gordon,J. Phys. Chem. 64, 1751 (1960).
M. Spiro,J. Chem. Phys. 42, 4060 (1965).
L. J. M. Smits and E. M. Duyvis,J. Phys. Chem. 70, 2747 (1966).
F. Culkin and J. P. Riley, inChemical Oceanography, J. P. Riley and G. Skirrow, eds. (Academic Press, London, New York, 1965), Chapter IV.
M. Périé, J. Périé, and M. Chemla,J. Chim. Phys. 65, 1284 (1968).
A. Poisson, Thesis, University of paris (1978).
M. Chemla,C. R. Acad. Sci., Ser. C 263, 184 (1966).
R. A. Robinson and R. H. Stokes,Electrolyte solutions, 2nd rev. edn. (Butterworths, London, 1965), p. 463.
M. Whitfield, Seawater as an electrolyte solution, inChemical Oceanography, 2nd ed., J. P. Riley and G. Skirrow, eds. (Academic Press, London, 1975), Chapter 2.
D. M. Connors and P. K. Weyl, Limnol. Oceanogr.13, 39 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Poisson, A., Périé, M., Périé, J. et al. Individual equivalent conductances of the major ions in seawater. J Solution Chem 8, 377–394 (1979). https://doi.org/10.1007/BF00646790
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00646790