Summary
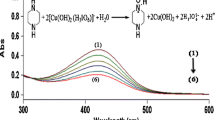
The kinetics of the oxidation of hypophosphite ion by platinum(IV) have been studied spectrophotometrically in alkaline medium at different temperatures. The rate increases as the pH increases and the empirical rate law applicable to the reaction is given by:-d[PtIV]/dt = k3[PtIV][H2PO2−][OH−]
The rate constant is 2.17×10−3 (l2 mo−2s−1) at 40.5°. The energy and entropy of activation for the reaction are 104.2 kJ mol−1 and 28.5 JK−1mol−1 respectively.
Similar content being viewed by others
References
U. S. Mehrotra, M. C. Agarwal and S. P. Mushran,J. Inorg. Nucl. Chem., 32, 2325 (1970).
A. A. Grinberg, and Yu N. Kakushkin,Dokl. Akad. Nauk., 140, 1976 (1961).
A. J. Poe and M. S. Vaidya,J. Chem. Soc., 2981 (1961).
K. G. Moodley and M. J. Nicol:J. Chem. Soc. Dalton Trans. 239 (1977).
K. K. Sen Gupta and P. K. Sen,J. Inorg. Nucl. Chem., 40, 1657 (1978)
V. Balzani and V. Carassiti,Photochemistry of Coordination Compounds, Academic Press, London, 1970, p. 258.
K. K. Sen Gupta, P. K. Sen and S. Sen Gupta,Inorg. Chem., 16, 1396 (1977).
K. K. Sen Gupta and P. K. Sen,J. Inorg. Nucl. Chem., 39, 1651 (1977).
K. K. Sen Gupta, S. Maiti and S. P. Ghosh,Ind. J. Chem., (accepted for publication).
K. K. Sen Gupta, J. K. Chakladar and A. K. Chatterjee:J. Inorg. Nucl. Chem., 35, 901 (1973)
R. L. Carrol and K. B. Thomas,J. Am. Chem. Soc., 1376 (1966)
J. N. Cooper, H. L. Hoyt, C. W. Buffington and C. A. Holmes,J. Phys. Chem., 75, 891 (1971)
E. Benzvi,Inorg. Chem., 6, 1143 (1967).
C. H. Bamford, A. D. Jenkins and R. Johnson;Nature, 177, 992 (1956)
E. Collinson and F. S. Dainton:Nature, 177, 1224 (1956)
C. Walling,Free radicals in Solutions, Wiley, New York, 1947, p. 520
P. M. Nave and W. S. Trahanovsky,J. Am. Chem. Soc., 93, 4536 (1971).
S. J. Paton and C. H. Brubaker,Inorg. Chem., 12, 1402 (1973).
R. L. Rich and H. Taube,J. Am. Chem. Soc., 76, 2608 (1954).
A. W. Adamson and A. H. Sporer,J. Am. Chem. Soc., 80, 3865 (1958).
J. Halpern and M. Pribanic,J. Am. Chem. Soc., 90, 5942 (1968).
A. Peloso and M. Basolo,Coord. Chem. Rev., 8, 111 (1972).
A. Peloso and M. Basolo,J. Chem. Soc., 725 (1971).
A. Peloso and M. Basolo,J. Chem. Soc. Dalton Trans., 2040 (1972).
A. A. Grinberg,The Chemistry of Complex Compounds, Pergamon Press, London, 1962, p. 279.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sen Gupta, K.K., Maiti, S. & Sen, P.K. Kinetics of oxidation of hypophosphite ion by platinum(IV) in an alkaline medium. Transition Met Chem 6, 264–266 (1981). https://doi.org/10.1007/BF00620743
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00620743