Summary
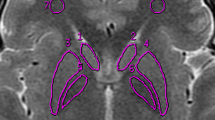
Heavily T2-weighted spin echo sequences of the brain show age-dependent low signal intensity in many extrapyramidal nuclei. Although it has been suggested that this low intensity results from non-haem iron, the specific influence of non-haem iron on the T2 relaxation time has not been quantified and remains controversial. The T2 relaxation times of the globus pallidus and putamen were measured from MRI at 1.5T in 27 healthy patients, by using a mathematical model. They were then plotted as a function of age and compared to the curve of age-dependent iron concentration determined post mortem. The curves of T2 relaxation time in the basal ganglia are congruent with published curves of iron concentration, indicating a high probability that the changes in T2 relaxation times and the low signal in the basal ganglia result from the local, age-dependent iron deposition. Individual measurements of T2 relaxation time show less variation before than after 45 years of age, indicating the influence of a second, more individual factor.
Similar content being viewed by others
References
McArdle CB, Richardson CJ, Nicholas DA, Mirfakhraee M, Hayden CK, Amparo EG (1987) Developmental features of the neonatal brain: MR imaging. I. Gray-white matter differentiation and myelination. Radiology 162: 223–229
Curnes JT, Burger PC, Djang WT, Boyko OB (1988) MR imaging of compact white matter pathways. AJNR 9: 1061–1068
Naidich TP, Daniels DL, Pech P, Haughton VM, Williams A, Pojunas K (1986) Anterior commissure: anatomic-MR correlation and use as a landmark in three orthogonal planes. Radiology 158: 421–429
Koenig SH, Brown RD (1986) Relaxometry of ferritin solutions and the influence of the Fe3+ core ions. Magn Reson Med 3: 755–767
Drayer BD (1989) Basal ganglia: significance of signal hypointensity on T2 weighted MR images. Radiology 173: 311–312
Drayer BP, Olanow W, Burger P, Johnson GA, Herfkens R, Riederer S (1986) Parkinson plus syndrome: diagnosis using high field MR imaging of brain iron. Radiology 159: 493–498
Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA (1986) MRI of brain iron. AJR 147: 103–110
Rutledge JN, Hilal SK, Silver AJ, Defendini R, Fahn S (1987) Study of movement disorders and brain iron by MR. AJNR 8: 397–411
Chen JC, Hardy PA, Clauberg M, et al (1989) T2 values in the human brain: comparison with quantitative assays of iron and ferritin. Radiology 173:521–526
Brooks DJ, Luthert P, Gadian D, Marsden CD (1989) Does signal-attenuation on high-field T2-weighted MRI of the brain reflect regional cerebral iron deposition? Observations on the relationship between regional cerebral water proton T2 values and iron levels. J Neurol Neurosurg Psychiatry 52:108–111
Hallgren B, Sourander P (1958) The effect of age on the nonhaemin iron in the human brain. J Neurochem 3:41–51
Höck A, Demmel U, Schicha H, Kasperek K, Feinendegen LE (1975) Trace element concentration in human brain. Brain 98: 49–64
Drayer B, Burger P, Hurwitz B, Dawson D, Cain J (1987) Reduced signal intensity on MR images of thalamus and putamen in multiple sclerosis: increased iron content? AJR 149: 357–363
Dietrich RB, Bradley WG (1988) Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology 168: 203–206
Gomori JM, Grossman RI, Goldberg HI, et al. (1985) Intracranial hematomas: Imaging by high-field MR. Radiology 157: 87–93
Bizzi A, Brooks RA, Brunetti A, et al (1990) Role of iron and ferritin in MR imaging of brain: a study in primates at different field strengths. Radiology 177:59–65
Rowe JW, Kahn RL (1987) Human aging: usual and successful. Science 237: 143–149
Drayer BD (1988) Imaging of the aging brain. I. Normal findings. Radiology 166: 785–796
Jorgensen L, Torvik A (1966) Ischaemic cerebrovascular diseases in an autopsy series. I. Prevalence, location and predisposing factors in verified thromboembolic occlusion, and their significance in pathogenesis of cerebral infarction. J Neurol Sci 3: 490–509
Breger RK, Rimm AA, Fischer ME, Papke RA, Haughton VM (1989) T1 and T2 measurements on a 1.5-T commercial MR imager. Radiology 171:273–276
Kjos BO, Ehman RL, Brant-Zawadzki M (1985) Reproducibility of T1 and T2 relaxation times calculated from routine MR imaging sequences: phantom study. AJNR 6:277–283
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schenker, C., Meier, D., Wichmann, W. et al. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology 35, 119–124 (1993). https://doi.org/10.1007/BF00593967
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00593967