Abstract
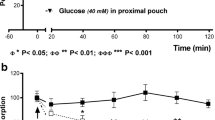
Experiments were carried out on 11 anesthetized cats (Na-pentobarbital). Uptake of a glucose analogue (2-deoxy-d-glucose) by intestinal mucosa and muscularis from arterial plasma was studied in order to determine net rate of transport and phosphorylation of the material. The theoretical basis for calculating the rate constants of forward (k x1 ) and reverse (k x2 ) transport between plasma and tissue and also phosphorylation (k x3 ) and dephosphorylation (k x4 ) of14C labeled deoxy-glucose (DG) was determined. The method can also be used for estimating tissue glucose uptake. The rate constants were found to be:k x1 =0.669 and 0.873;k x2 =2.285 and 4.656;k x3 =0.057 and 0.067;k x4 =0.091 and 0.097 [s−1] in the mucosa and muscularis, respectively. Glucose utilisation of intestinal mucosa was 2.69 and that of muscularis 2.46 mg/(min×100 g) tissue, respectively. Arterial glucose concentration was constant during the studies, however, it showed a variation from 120 to 250 mg/100 ml of plasma from animal to animal. Tissue glucose uptake or any of the rate constants were not influenced by the plasma level over this range.
Similar content being viewed by others
References
Alteveer RJ, Goldfarb RD, Lau J, Port M, Spitzer JJ (1973) Effect of severe acute hemorrhage on metabolism of the dog intestine. Am J Physiol 224:197–201
Anderson JW (1974) Glucose metabolism in jejunal mucosa of fed, fasted and streptozotocin-diabetic rats. Am J Physiol 226:226–229
Bergmeyer HM (1962) Bestimmung mit Glucose-Oxydase und Peroxydase. In: Methoden der enzymatischen Analyse. Verlag Chemie GmbH, Weinheim, S 123–130
Clark B, Sherratt HSA (1967) Glycolysis and oxydations in preparations from small-intestinal mucosa of four species. Comp Biochem Physiol 20:223–243
Crane RK, Sols A (1953) The association of hexokinase with particulate fractions of brain and other tissue homogenates. J Biol Chem 203:273–279
Esposito G, Csaky TZ (1974) Extracellular space in the epithelium of rat's small intestine. Am J Physiol 226:50–55
Hamar J, Polenov AS, Tcherniavskaja GV, Berezina TP, Dézsi L (1981) Effect of hypoxia on microcirculation and energy supply of the small intestine in cats. In: Kovách AGB, Dóra E, Kessler M, Silver IA (eds) Oxygen transport to tissue. Advances in physiological sciences, vol 25. Akadémiai Kiadó, Pergamon Press, Budapest, pp 173–174
Hers M (1957) La metabolism du fructose, Abscisa. Bruxelles, p 102
Hohenleithner FJ, Senior JR (1969) Metabolism of canine small intestine vascularly perfused in vitro. J Appl Physiol 26:119–128
Holt PR, Haessler HA, Isselbachet KJ (1963) Effects of bile salts on glucose metabolism by slices of hamster small intestine. J Clin Invest 42:777–786
Hübscher G, West GR, Brindley DN (1965) Studies on the fractionation of mucosal homogenates from the small intestine. Biochem J 97:629–642
Klendshoj NC, Feldstein M, Spragne AL (1950) The spectrophotometric determination of carbon monoxyde. J Biol Chem 183:297–303
Landau BR, Lubbs HA (1958) Animal responses to 2-deoxy-d-glucose administration. Proc Soc Exp Biol Med 99:124–127
Lea MA, Walker DG (1964) The metabolism of glucose-6-phosphatase in developing mammalian tissues. Biochem J 91:417–424
Long C (1951) Studies involving enzymic phosphorylation. Biochem J 50:407–415
Lygre DG, Nordlie RC (1969) Studies on the fractionation of mucosal homogenates from the small intestine. Biochim Biophys Acta 185:360–366
Öckerman PA (1965) Glucose-6-phosphatase in human jejunal mucosa; properties demonstrating the specific character of the enzyme activity. Biochim Biophys Acta 105:22–33
Port M (1971) Metabolism of free fatty acids and glucose in the small intestine of the dog. Thesis. Faculty of Medicine, Hahneman Med Coll, Philadelphia
Raggi F, Kronfeld DS, Kleiberg M (1960) Glucose-6-phosphatase activity in various sheep tissues. Proc Soc Exp Biol Med 105:485–486
Reivich M, Sano N, Sokoloff L (1971) Development of an autoradiographic method for the determination of regional cerebral glucose consumption. In: Ross Russel RW (ed) Brain and blood flow. Pittman Medical and Scientific Publishing Co. Ltd., London, pp 397–400
Roese HF (1930) Methoden zum Studium der Physiologie und Pharmacologie des künstlich durchbluteten Säugetierdarmes. Pflügers Arch Ges Physiol 226:171–183
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettegrew KD, Sakurada O, Shinohara M (1977) The14C-deoxyglucose method for the measurement of local cerebral glucose utilisation: Theory, procedure and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Spitzer JJ, Nakamura H, Hori S, Gold M (1919) Hepatic and splanchnic uptake and oxydation of free fatty acids. Proc Soc Exp Biol Med 132:281–286
Srivastava LM, Hübscher G (1966) Glucose metabolism in the mucosa of the small intestine: Glycolysis in subcellular preparations from cat and rat. Biochem J 100:458–466
Tower D (1958) The effect of 2-deoxy-d-glucose on metabolism of slices of cerebral cortex in vitro. J Neurochem 3:185–205
Wick AN, Drury DR, Nakada HI, Wolfe JB (1957) Localisation of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem 224:963–969
Woodward GE, Hudson MT (1954) The effect of 2-deoxy-d-glucose on glycolysis and respiration of tumor and normal tissues. Cancer Res 14:599–605
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hamar, J., Hutiray, G. In vivo determination of transport and metabolism of deoxyglucose in intestinal tissues. Pflugers Arch. 401, 233–238 (1984). https://doi.org/10.1007/BF00582589
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00582589