Summary
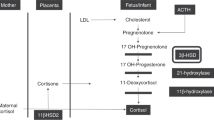
Corticosteroids (CS) are known to be essential for fetal organ maturation and seem to play an important role in both the initiation of parturition and the postnatal adaptation of the human neonate. Pharmacologically, CS are widely used for enhancing fetal lung maturation prior to premature delivery. However, knowledge of endogenous CS and precursor levels throughout fetal and perinatal life and their response to exogenous CS is limited. Therefore, using automated liquid column chromatography plus specific radioimmunoassays, unconjugated aldosterone (Aldo), corticosterone (B), 11-deoxycorticosterone (DOC), progesterone (P), 17-hydroxyprogesterone (17-OHP), 11-deoxycortisol (S), cortisol (F) and cortisone (E) were simultaneously followed in 70 amniotic fluid (AF) control samples throughout pregnancy, and in cord and neonatal plasma longitudinally during the first week of life. From 14 to 38 weeks, AF levels of all measured steroids except E rose by 2 to 12-fold on the average (allP<0.001) but declined at term. E increased until 31–35 weeks (P<0.01), then remained almost constant until term. Cord levels of all steroids were substantially higher than those found in AF at term. While levels of the placentally derived steroids P, 17-OHP, DOC and E dropped sharply after birth by several orders of magnitude (P≪0.01) showing typical disappearance curves, the biologically most potent CS Aldo and F rose even further immediately after birth. Whereas Aldo levels declined from maxima about 100 times above normal adult levels at 6 h by almost 3-fold until day 7 (P<0.01), F (and also B) fluctuated considerably resembling a damped oscillation and, by day 7, reached mean levels less than half of those seen in later childhood. After betamethasone treatment of the mother, neonatal levels of Aldo and F were suppressed to 24–69% of normal until day 9, whereas those of the other steroids (except E) returned to normal during the first hours of life. Phenobarbital (PB) therapy of the mother led to decreased steroid levels in maternal and umbilical venous plasma at term, while umbilical arterial CS levels, notably those of Aldo and F (P<0.02), were increased when compared with untreated controls, indicating a stimulation of the most potent CS in the fetus after PB. The significance of the findings in view of fetoplacental function and fetal organ maturation is briefly discussed.
Similar content being viewed by others
References
Johannisson E (1968) The foetal adrenal cortex in the human. Its ultrastructure at different stages of development and in different functional states. Acta Endocrinol (Copenhagen) 58: [Suppl 130] 7–107
Diczfalusy E (1969) Steroid metabolism in the human foetoplacental unit. Acta Endocrinol (Copenhagen) 61: 649–664
Solomon S, Friesen HG (1968) Endocrine relations between mother and fetus. Annu Rev Med 19: 399–430
Pasqualini JR, Wiqvist N, Diczfalusy E (1966) Biosynthesis of aldosterone by human foetuses perfused with corticosterone at mid-term. Biochim Biophys Acta 121: 430–431
Dufau ML, Villee DB (1969) Aldosterone biosynthesis by human fetal adrenal in vitro. Biochim Biophys Acta 176: 637–640
Seron-Ferre M, Lawrence CC, Siiteri PK, Jaffe RB (1978) Steroid production by definitive and fetal zones of the human fetal adrenal gland. J Clin Endocrinol Metab 47: 603–609
Sippell WG, Bidlingmaier F, Becker H, Brünig T, Dörr H, Hahn H, Golder W, Hollmann G, Knorr D (1978) Simultaneous radioimmunoassay of plasma aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, 11-deoxycortisol, cortisol and cortisone. J Steroid Biochem 9: 63–74
Liggins GC, Howie RN (1972) A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50: 515–525
Trølle D (1976) Decrease in the mortality rates for low-birth-weight infants after phenobarbitone treatment. Acta Obstet Gynecol Scand 55: 13–20
Sippell WG, Becker H, Versmold HT, Bidlingmaier F, Knorr D (1978) Longitudinal studies of plasma aldosterone, corticosterone, deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone determined simultaneously in mother and child at birth and during the early neonatal period. I. Spontaneous delivery. J Clin Endocrinol Metab 46: 971–985
Sippell WG, Bidlingmaier F, Knorr D (1978) Mechanized Sephadex LH-20 multiple column chromatography as a prerequisite for automated multisteroid radioimmunoassays. In: Radioimmunoassay and related procedures in medicine 1977, Vol I. International Atomic Energy Agency, Vienna, pp 229–238
Sachs L (1978) Angewandte Statistik, 5. Aufl. Springer, Berlin Heidelberg New York
Sippell WG, Dörr HG, Bidlingmaier F, Knorr D (1980) Plasma levels of aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone during infancy and childhood. Pediatr Res 14: 39–46
Abramovich DR, Wade AP (1969) Transplacental passage of steroids: The presence of corticosteroids in amniotic fluid. J Obstet Gynaecol Br Commonw 76: 610–614
Schweitzer M, Klein GP, Giroud CJP (1971) Characterization of 17-deoxy-and 17-hydroxycorticosteroids in human liquor amnii. J Clin Endocrinol Metab 33: 605–611
Murphy BEP, Patrick J, Denton RL (1975) Cortisol in amniotic fluid during human gestation. J Clin Endocrinol Metab 40: 164–167
Murphy BEP (1978) Conjugated glucocorticoids in amniotic fluid and fetal lung maturation. J Clin Endocrinol Metab 47: 212–215
Bayard F, Ances IG, Tapper AJ, Weldon VV, Kowarski A, Migeon CJ (1970) Transplacental passage and fetal secretion of aldosterone. J Clin Invest 49: 1389–1393
Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ (1973) The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res 7: 509–519
Tuimala R, Kauppila A, Haapalahti J (1976) ACTH levels in amniotic fluid during pregnancy. Br J Obstet Gynaecol 83: 853–856
Skinner SL, Cran EJ, Gibson R, Taylor R, Walters WAW, Catt KJ (1975) Angiotensins I and II, active and inactive renin, renin substrate, renin activity, and angiotensinase in human liquor amnii and plasma. Am J Obstet Gynecol 121: 626–630
Murphy BEP (1977) Chorionic membrane as an extra-adrenal source of foetal cortisol in human amniotic fluid. Nature 266: 179–181
Liggins GC (1976) Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol 126: 931–941
Challis JRG, Thorburn GD (1975) Prenatal endocrine function and the initiation of parturition. Br Med Bull 31: 57–62
Sivakumaran T, Duncan ML, Effer SB, Younglai EV (1975) Relationship between corticol and lecithin/sphingomyelin ratios in human amniotic fluid. Am J Obstet Gynecol 122: 291–294
Gewolb IH, Hobbins JC, Tan SY (1977) Amniotic fluid cortisol as an index of fetal lung maturity. Obstet Gynecol 49: 462–465
Ballard PL, Ballard RA (1979) Corticosteroids and respiratory distress syndrome: Status 1979. Pediatrics 63: 163–165
Ballard PL, Granberg P, Ballard RA (1975) Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest 56: 1548–1554
Sybulski S (1977) Effect of antepartum betamethasone treatment on cortisol levels in cord plasma, amniotic fluid, and the neonate. Am J Obstet Gynecol 127: 871–874
Kauppila A, Koivisto M, Pukka M, Tuimala R (1978) Umbilical cord and neonatal cortisol levels. Effect of gestational and neonatal factors. Obstet Gynecol 52: 666–672
Ohrlander S, Gensser G, Nilsson KO, Eneroth P (1977) ACTH test to neonates after administration of corticosteroids during gestation. Obstet Gynecol 49: 691–694
Burstein S, Klaiber EL (1965) Phenobarbital-induced increase in 6β-hydroxycortisol excretion: Clue to its significance in human urine. J Clin Endocrinol Metab 25: 293–296
Welch RM Harrison YE, Gommi BW, Poppers PJ, Finster M, Conney AH (1969) Stimulatory effect of cigarette smoking on the hydroxylation of 3,4-benzpyrene and the N-demethylation of 3-methyl-4-monomethylaminoazobenzene by enzymes in human placenta. Clin Pharmacol Ther 10: 100–109
Reynolds JW, Mirkin BL (1973) Urinary corticosteroid and diphenylhydantoin metabolite patterns in neonates exposed to anticonvulsant drugs in utero. Clin Pharmacol Ther 14: 891–897
Remmer R (1977) Personal communication.
Author information
Authors and Affiliations
Additional information
Supported by a grant from the German Federal Ministry for Research and Technology (BMFT)
Rights and permissions
About this article
Cite this article
Sippell, W.G., Bidlingmaier, F. & Knorr, D. Development of endogenous glucocorticoids, mineralocorticoids and progestins in the human fetal and perinatal period. Eur J Clin Pharmacol 18, 95–104 (1980). https://doi.org/10.1007/BF00561485
Issue Date:
DOI: https://doi.org/10.1007/BF00561485