Summary
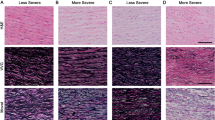
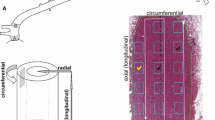
The ultrastructural organization of the adult human aortic media was studied utilizing aortic biopsies from 14 patients, ranging in age from 28 to 67, who underwent cardiac surgery. Apart from solid elastic elements the tissue spaces contained a vast amount of ill-defined thin streaks of elasin, an observation much facilitated by utilizing a selective elastin staining technique. In favorable sections, these streaks were found to be continuous with the solid elastic laminae. Furthermore, most medial smooth muscle cells were in close contact with the thin streaks, but almost none directly with the elastic laminae. The smooth muscle cells had also virtually no connection with collagen fibers. These observations are in contrast with the organization of elastin and with cell-to-stroma connections in the more extensively studied rodent and porcine aortas; they bring into question the role of the smooth muscle cells in the regulation of the viscoelastic properties of the human aortic wall. Other findings were: large number of nexuses connecting the smooth muscle cells, a very small degree of smooth muscle cell degeneration, and the presence of flocculent, fine-granular material investing all formed elements, but especially associated with the thin streaks of elastin.
Similar content being viewed by others
References
Bierring F, Kobayasi T (1963) Electron microscopy of the normal rabbit aorta. Acta Pathol Microbiol Scand 57:154–168
Clark JM, Glagov S (1979) Structural integration of the arterial wall. I. Relationships and attachments of medial smooth muscle cells in normally distended and hyperdistended aortas. Lab Invest 40:587–602
Cliff WJ (1967) The aortic tunica media in growing rats studied with the electron microscope. Lab Invest 17:599–615
Cliff WJ (1970) The aortic tunica media in aging rats. Exp Mol Pathol 13:172–189
Dingemans KP, Lettinga KP (1976) Modifications to an LKB Pyramitome. J Microsc (Oxford) 108:301–306
Ferrans VJ, Roberts WC (1976) The carcinoid endocardial plaque. An ultrastructural study. Hum Pathol 7:387–409
Gabella G (1972) The arrangement of sarcoplasmic reticulum in smooth muscle. Experientia 28:948–949
Gabella G (1975) Hypertrophy of intestinal smooth muscle. Cell Tissue Res 163:199–214
Gabella G (1976) Structural changes in smooth muscle cells during isotonic contraction. Cell Tissue Res 170:187–201
Gabella G (1977) Arrangement of smooth muscle cells and intramuscular septa in the taenia coli. Cell Tissue Res 184:195–212
Gerrity RG, Cliff WJ (1975) The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest 32:585–600
Haust MD (1965) Fine fibrils of extracellular space (microfibrils). Their structure and role in connective tissue organization. Am J Pathol 47:113–1137
Haust MD, More RK, Bencosme SA, Balis JU (1965) Elastogenesis in human aorta: an electron microscopic study. Exp Mol Pathol 4:508–524
Henderson RM, Duchon G, Daniel EE (1971) Cell contacts in duodenal smooth muscle layers. Am J Physiol 221:564–574
Herr JC (1976) Reflexive gap junctions. Gap junctions between processes arising from the same ovarian decidual cell. J Cell Biol 69:495–501
Imai H, Lee SK, Pastori SJ, Thomas WA (1970) Degeneration of arterial smooth muscle cells: ultrastructural study of smooth muscle cell death in control and cholesterol-fed animals. Virchows Arch [Pathol Anat] 350:163–204
Joris I, Majno G (1974) Cellular breakdown within the arterial wall. An ultrastructural study of the coronary artery in young and aging rats. Virchows Arch [Pathol Anat] 364:111–127
Kádár A (1974) The ultrastructure of elastic tissue. Pathol Europ 9:133–146
Kádár A, Gardner DL, Bush V (1972) Glycosaminoglycans in developing chick-embryo aorta revealed by ruthenium red: an electron microscope study. J Pathol 108:275–280
Kajikawa K, Yamaguchi T, Katsuda S, Miwa A (1975) An improved electron stain for elastic fibers using tannic acid. J Electron Microsc 24:287–289
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A
Karrer H, Cox J (1961) An electron microscope study of the aorta in young and aging mice. J Ultrastruct Res 5:1–27
Kim KM (1976) Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc 35:156–162
Knieriem HJ, Hueber R (1970) Quantitative morphologische Untersuchungen an der Aorta des Menschen. Beitr Path Anat 140:280–297
Lane BP (1965) Alterations in the cytologic detail of intestinal smooth muscle cells in various stages of contraction. J Cell Biol 27:199–213
Massmann J, Weidenbach H (1975) Lichtmikroskopische und elektronenoptische Untersuchungen zur spontanen kalzifizierenden Mediasklerose des Kaninchens. Zentralbl Allg Pathol 119:179–188
Morgan AJ (1980) Mineralized deposits in the thoracic aorta of aged rats: ultrastructural and electron probe X-ray microanalysis. Exp Geront 15:563–573
Paule WJ (1963) Electron microscopy of the newborn rat aorta. J Ultrastruct Res 8:219–235
Race GJ (1971) In Atlas of human histology and ultrastructure, Matthews JL, Martin JH (eds). Lea & Febiger Philadelphia
Riede UN, Staubesand J (1977) A unifying concept for the role of matrix vesicles and lysosomes in the formal pathogenesis of diseases of connective tissues and blood vessels. Beitr Path 160:3–37
Ross R (1973) The leastic fiber. A review. J Histochem Cytochem 21:199–208
Schlatmann TJM, Becker AE (1977) Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol 39:13–20
Seifert K (1962) Elektronenmikroskopische Untersuchungen der Aorta des Hausschweines. Z Zellforsch 58:331–368
Seifert K (1963) Elektronenmikroskopische Untersuchungen der Aorta des Kaninchens. Z Zellforsch 60:293–312
Simionescu N, Simionescu M (1976) Galloylglucoses of low molecular weights as mordant in electron microscopy. I. Procedure and evidence for mordanting effect. J Cell Biol 70:608–621
Stein I, Eisenberg S, Stein Y (1969) Aging of aortic smooth muscle cells in rats and rabbits: a morphologic and biochemical study. Lab Invest 21:386–397
Theman TE, Silver MD, Haust MD, McLoughlin MJ, Wigle ED, Williams WR (1979) Morphological findings in idiopathic calcification of the ascending aorta and aortic valve affecting a young woman. Histopathology 3:181–190
Thyberg J, Hinek A, Nilsson J, Friberg U (1979) Electron microscopic and cytochemical studies of rat aorta. Intracellular vesicles containing elastin- und collagen-like material. Histochem J 11:1–17
Trillo A, Haust MD (1975) The granulovesicular bodies of the arterial wall. Lab Invest 32:105–110
Veltman E, Backwinkel KP, Themann H, Hauss WH (1975) Elektronmikroskopische Untersuchungen zur Entstehung von “ghost bodies” in Aorten. Virchows Arch [Pathol Anat] 367:281–288
Wight TN, Ross R (1975) Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol 67:660–674
Wolinsky H, Glagov S (1967) A lamellar unit of aortic medial structure and function in mammals. Circ Res 20:99–111
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dingemans, K.P., Jansen, N. & Becker, A.E. Ultrastructure of the normal human aortic media. Virchows Arch. A Path. Anat. and Histol. 392, 199–216 (1981). https://doi.org/10.1007/BF00430821
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00430821