Summary
Trirhabda bacharidis (Weber) (Chrysomelidae), a univoltine, monophagous beetle is the dominant herbivore on Baccharis halimifolia (Compositae), a woody, perennial shrub that leafs out in early spring and retains its leaves into November. Available plant biomass increases during the season but T. bacharidis feeds only during spring and early summer. During the remainder of the growing season, there are no major herbivores feeding on B. halimifolia.
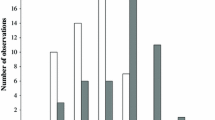
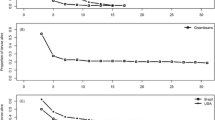
B. halimifolia leaves increase in toughness and thickness and decrease in moisture and nutrients as the season progresses. In feeding preference tests, T. bacharidis larvae preferred young leaves over leaves of older age classes. When reared on leaves of different ages, larvae fed on young leaves, weighed more, pupated earlier and had greater survivorship. T. bacharidis larvae showed no significant feeding preference for similarly tough B. halimifolia leaves painted with differing concentrations of late season acetone leaf extract. Elm leaves painted with the same leaf extracts were avoided by larvae of the non-adapted specialist Pyrrhalta luteola (Mueller) (Chrysomelidae).
These results suggest that the adapted specialist, T. bacharidis, is not deterred by the B. halimifolia acetone soluble secondary chemical which increases in amount over the season. Decreasing leaf nitrogen (perhaps in concert with increasing leaf toughness) seems to be the primary factor that dissuades its feeding. However, acetone soluble secondary chemicals in the leaves of B. halimifolia may be effective in preventing herbivory from non-adapted insects.
Similar content being viewed by others
References
Anthonsen T, Bruun T, Hemmer E, Holme D, Lamvik A, Sunde E, Sorenson NA (1970) Baccharis oxide, a new triterpenoid from Baccharis halimifolia. Acta Chem Scandinavica 24:2479–2488
Auclair JL (1976) Feeding and nutrition of the pea aphid, Acyrthosiphon pisum (Harris), with special reference to amino acids. Symp Biol Hung 16:29–34
Blau PA, Feeny P, Contardo L (1970) Allylglucosinolate and herbivorous caterpillars: A contrast in toxicity and tolerance. Science 200:1296–1298
DaCosta CP, Jones CM (1971) Cucumber beetle resistance and mite susceptibility controlled by the bitter gene in Cucumis sativus L. Science 172:1145–1146
Denno RF, Raupp MJ, Tallamy DW, Reichelderfer CF (1980) Migration in heterogeneous environments: Differences in habitat selection between the wing-forms of the dimorphic planthopper, Prokelisia marginata (Homoptera: Delphacidae). Ecology 61:859–867
Dethier VG (1954) Evolution of feeding preference in phytophagous insects. Evolution 8:33–54
Dixon AFG (1971) The role of aphids in wood formation. I. The effect of the sycamore aphid, Drepanosiphum platanoides (Schr). (Aphididae), on the growth of sycamore, Acer pseudoplatanus (L.). J Appl Ecol 8:165–179
Feeny P (1970) Seasonal changes in the oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Feeny P (1975) Biochemical coevolution between plants and their insect herbivores. Coevolution of Animals and Plants in: LE Gilbert and PH Raven (eds), University of Texas Press Austin p 3–19
Feeny P (1976) Plant apparency and chemical defense. Biochemical interaction between plants and insects Vol 10, Recent advances in phytochemistry in: J Wallace and RL Mansell (eds), Plenum Press New York, p 1–40
Glander KE (1975) Habitat and resource utilization: An ecological view of social organization in manteled howling monkeys, Dissertation, University of Chicago Chicago
Horsfield D (1977) Relationships between feeding of Philaenus spumarius (L) and the amino acid concentration in the xylem sap. Ecol Entomol 2:259–266
Horwitz W (ed) (1965) Official methods of analysis of the association of official agricultural chemists, 10th edition. AOAC Washington DC
Johnson WT, Lyon HH (1976) Insects that feed on trees and shrubs. Comstock Associates, Cornell University Press Ithaca
Lavie D, Jain MK, Shpan-Gabrielith SR (1967) A locust phagorepellent from two Melia species. Chem Commun 910–911
Lawton JH (1976) The structure of the arthropod community on bracken. Bot J Linn Soc 73:187–216
Mabry TJ, Gill JE (1979) Sesquiterpene lactones and other terpenoids. Herbivores, their interaction with secondary plant metabolites. In: GA Rosenthal and DH Janzen (eds), Academic Press New York, p 502–533
McNeill S, Southwood TRE (1978) The role of nitrogen in the development of insect/plant relationships. Biochemical aspects of plant and animal coevolution in: JB Harbone (ed), Proceedings of the Phytochemical Society Symposium p 78–98
Mo F (1973) Crystal structure of the triterpenoid Baccharis oxide: with a note on the use of various approaches in the tangent-refinement procedure. Acta Crystall Sect B, Struct Crystall and Crystal Chem 29:1796–1807
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. Herbivores, their interaction with secondary plant metabolites. In: GA Rosenthal and DH Janzen (eds), Academic Press New York, p 4–48
Rhoades DF, Cates RG (1976) Toward a general theory of plant antiherbivore chemistry. Biochemical interaction between plants and insects Vol 10, Recent advances in phytochemistry. In: JW Wallace and RL Mansell (eds), Plenum Press, New York, p 168–213
Rockwood LL (1973) The effect of defoliation on seed production of six Costa Rican tree species. Ecology 54:1363–1369
Rockwood LL, Glander KE (1979) Howling monkeys and leafcutting ants: Comparative foraging in a tropical deciduous forest. Biotropica 11:1–10
Schweizer CJ, Ries SK (1969) Protein content of seeds: increase improves growth and yield. Science 165:73–75
Tanton MT (1962) The effect of leaf “toughness” on the feeding of larvae of the mustard beetle Phaedon cochleariae Fab. Entomol Exper et Appl 5:74–78
Westman WE, Panetta FD, Stanley TD (1975) Ecological studies on reproduction and establishment of the woody weed, groundsel bush (Baccharis halimifolia L.: Asteraceae) Australian J Agric Res 26:855–870
Williams LH (1954) The feeding habits and food preferences of Acrididae and the factors which determine them. Trans Royal Entomol Soc London 105:423–454
Woodwell GM, Whittaker RH, Houghton RA (1975) Nutrient concentrations in plants in the Brookhaven oak-pine forest. Ecology 56:318–332
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kraft, S.K., Denno, R.F. Feeding responses of adapted and non-adapted insects to the defensive properties of Baccharis halimifolia L. (Compositae). Oecologia 52, 156–163 (1982). https://doi.org/10.1007/BF00363829
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00363829