Abstract
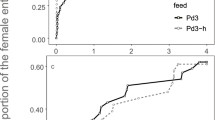
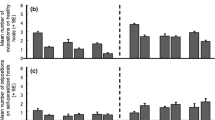
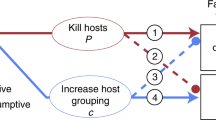
Previous studies on frequency-dependent food selection (changing food preferences in response to changes in relative food abundance) have focused on predators and parasitoids. These organisms utilize several victims during their lifetime. We introduce the case of parasites which, having accepted a host, do not change it. We propose two alternative models to explain the biased occurrence of parasites on different host types: (1) through the option of rejecting less-preferred hosts prior to accepting one of them; (2) by differential parasite survival on different host types. These models predict that host rejection, but not differential survival, can create frequency-dependent parasitism (FDP). Unlike previously described factors responsible for frequency dependence of food selection, which act through changing the foraging behaviour of individual predators or parasitoids, FDP involves no adjustment of parasite foraging strategy according to previous feeding experience. The mite Hemisarcoptes coccophagus is an obligate parasite of armoured scale insects (Homptera: Diaspididae). Our field data show that H. coccophagus is found more frequently on ovipositing than on young host females. Our model, combining the effects of host rejection and differential survival, is used to estimate the relative contribution of these factors to parasite biased occurrence on different hosts. The contribution of differential survival was dominant in H. coccophagus, and overode any effect of host rejection. Nevertheless, our prediction that FDP may be found in parasites is supported by literature data about a parasitic water mite.
Similar content being viewed by others
References
Abrams P, Matsuda H (1993) Effects of adaptive predatory and anti-predator behaviour in a two-prey-one-predator system. Evol Ecol 7:312–326
Akre BG, Johnson DM (1979) Switching and sigmoid functional response curves by damselfly naiads with alternative prey available. J Anim Ecol 48:703–720
Allen JA (1988) Frequency-dependent selection by predators. Philo Trans R Soc B 319:485–503
Ayala FJ, Campbell CA (1974) Frequency-dependent selection. Annu Rev Ecol Syst 5:115–138
Begon M, Harper JL, Townsend CR (1990) Ecology: individuals, populations and communities. Blackwell, Oxford
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theor Popul Biol 9:129–136
Chesson J (1978) Measuring preference in selective predation. Ecology 59:211–215
Chesson J (1983) The estimation and analysis of preference and its relationship to foraging models. Ecology 64:1297–1304
Clarke B (1962) Balanced polymorphism and the diversity of sympatric species. In: Nichols D (ed) Taxonomy and geography, vol 4. (Systematics Association Publication). Academic Press, London, pp 47–50
Cock MJW (1978) The assessment of preferences. J Anim Ecol 47: 805–816
Davids C (1973) The water mite Hydrachna conjecta Koenike, 1895 (Acari, Hydrachnellae), bionomics and relation to species of Corixidae (Hemiptera). Neth J Zool 23:363–429
Elton RA, Greenwood JJD (1970) Exploring apostatic selection. Heredity 25:629–633
Endler JA (1988) Frequency-dependent predation, crypsis and aposematic coloration. Philos Trans R Soc B 319:505–523
Gerson U (1967) Interrelationships of two scale insects on citrus. Ecology 48:872–873
Gerson U, O'Connor BA, Houck MA (1990) Acari. In: Rosen D (ed) Armored scale insects: their biology, natural enemies and control, vol 4 B. Elsevier, Amsterdam, pp 77–97
Greenwood JJD (1984) The functional significance of frequency-dependent food selection. Biol J Linn Soc 23:177–199
Greenwood JJD, Elton RA (1979) Analysing experiments on frequency-dependent selection by predators. J Anim Ecol 48: 721–737
Hassell MP (1978) The dynamics of arthropod predator-prey association. Princeton University Press, Princeton
Hubbard SF, Cook RM, Glover JG, Greenwood JJD (1982) Apostatic selection as an optimal foraging strategy. J Anim Ecol 51:625–633
Hughes RN, Croy MI (1993) An experimental analysis of frequency-dependent predation (switching) in the 15-spined stickleback, Spinachia spinachia. J Anim Ecol 62:341–352
Izraylevich S, Gerson U (1993) Mite parasitization on armored scale insects: host suitability. Exp Appl Acarol 17:861–875
Lindquist EE (1983) Some thoughts on the potential for use of mites in biological control, including a modified concept of “parasitoids”. In: Hoy MA, Cunningham GL, Knutson L (eds) Biological control of pests by mites. Berkeley, California, pp 12–20
Manly BFJ (1973) A linear model for frequency-dependent selection by predators. Res Popul Ecol 14:137–150
Manly BFJ (1974) A model for certain types of selection experiments. Biometrics 30:281–294
Manly BFJ, Miller P, Cook LM (1972) Analysis of a selective predation experiment. Am Nat 106:719–736
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability on prey populations. Ecol Monogr 39:335–354
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Real LA (1990) Predator switching and the interpretation of animal choice behavior: the case for constrained optimization. In: Hughes RN (ed) Behavioural mechanisms of food selection. Springer, Berlin Heidelberg New York, pp 1–21
SAS (1988) SAS/STAT user's guide, 6.03 edn. SAS Institute, Cary
Sherratt TN, Harvey IF (1993) Frequency-dependent food selection by arthropods: a review. Biol J Linn Soc 48:167–186
Smith BP (1988) Host-parasite interaction and impact of larval water mites on insects. Annu Rev Entomol 33:487–507
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Izraylevich, S., Hasson, O. & Gerson, U. Frequency-dependent host selection by parasitic mites: a model and a case study. Oecologia 102, 138–145 (1995). https://doi.org/10.1007/BF00333244
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00333244