Summary
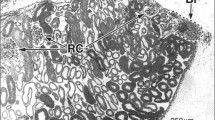
The construction of renal lobules in Triturus (Cynops) pyrrhogaster was studied by reconstruction from serial semithin sections, and the structure of nephrons, collecting ducts and ureters was investigated by means of light and electron microscopy. In T. pyrrhogaster the kidney was mesonephros in construction; renal lobules were arranged segmentally and each of them sent one ureter. Male ureters ran caudally and met together before joining the Wolffian duct. In renal lobules, long collecting ducts ran medio-laterally in the dorsal aspect of the kidney and sent several branches ventrally. Each branch duct or short collecting duct received one nephron. Each nephron had five segments; 1) renal corpuscle, 2) ciliated neck segment with or without a naphrostome, 3) proximal tubule, 4) ciliated intermediate segment and 5) distal tubule. Proximal and distal tubules were segregated spacially in renal lobules and occupied the peripheral and central zone respectively. The filtration barrier of the glomerulus consisted of both the basal lamina of podocytes and the subendothelial connective tissue, and was much thicker than the mammalian filtration barrier. Proximal tubule cells had a brush border, apical specialzation for reabsorption of organic materials and well-developed smooth endoplasmic reticulum, but few baso-lateral interdigitations. In distal tubule cells, baso-lateral interdigitations and infoldings were well-developed. Collecting duct cells had a sparse cytoplasm. Ureter cells in males contained many secretory granules. On the basis of structural organization of the newt kidney as well as physiological data in literature, we suggest that in land vertebrates proximal tubules were primarily adapted to reabsorption of organic materials and distal tubules to reabsorption of electrolytes and water.
Similar content being viewed by others
References
Bargmann W (1978) Harn-und Geschlechtsapparat. In Handbuch der mikroskopischen Anatomie des Menschen. (Möllendorff Wv/Bargmann W ed) VII/5, Springer, Berlin
Bargmann W, Knoop A, Schiebler ThH (1955) Histologische, cytochemische und elektronenmikroskopische Untersuchungen am Nephron (mit Berücksichtigung der Mitochondrien). Z Zellforsch 42:386–422
Bott PA (1962) Micropuncture study of renal excretion of water, K, Na, and Cl in Necturus. Am J Physiol 203:662–666
Bulger RE, Trump BF (1968) Renal morphology of the English sole (Parophrys vetulus). Am J Anat 123:195–226
Cardell RR, Badenhausen S, Porter KR (1967) Intestinal triglyceride absorption in the rat: an electron microscopic study. J Cell Biol 34:123–155
Chase SW (1923) The mesonephros and urogenital ducts of Necturus maculosus, Rafinesque. J Morphol 37:457–531
Clothier RH, Worley RTS, Balls M (1978a) A study of the renal tubule of the urodele amphibian Amphiuma means. J Anat 126:405–406
Clothier RH, Worley RTS, Balls M (1978b) The structure and ultrastructure of the renal tubules of the urodele amphibian, Amphiuma means. J Anat 127:491–504
Colbert EH (1980) Evolution of the vertebrates: a history of the backboned animals through time. 3rd ed. Wiley & Sons, New York
Davis LE, Schmidt-Nielsen B (1967) Ultrastructure of the crocodile kidney (Crocodylus acutus) with special reference to electrolyte and fluid transport. J Morphol 121:255–276
Fawcett DW (1981) The cell. 2nd ed. Saunders, Philadelphia
Frangioni G, Borgioli G (1980) Lactate sensitive cells in newt kidneys. Cell Tissue Res 207:233–239
Gegenbaur C (1901) Vergleichende Anatomie der Wirbeltiere mit Berücksichtigung der Wirbellosen. vol 2. Wilhelm Engelmann, Leipzig
Gérald P, Cordier R (1932) Études histophysiologiques sur le rein des anoures. Arch Biol Liege 43:367–413
Gérald P, Cordier R (1934) Esquisse d'une histophysiologie comparée du rein des vertébrés. Biol Rev Cambridge Phil Soc 9:110–131
Gibley CW, Chang JP (1967) Fine structure of the functional mesonephros in the eight-day chick embryo. J Morphol 123:441–462
Giebisch G, Windhager EE (1973) Electrolyte transport across renal tubular membranes. In: Orloff J, Berliner RW (ed) Handbook of physiology, section 8: Renal physiology. 315–376, American Physiological Society, Washington
Hanner RH, Ryan GB (1980) Ultrastructure of the renal juxtaglomerular complex and peripolar cells in the axolotl (Ambystoma mexicanum) and toad (Bufo marinus). J Anat 130:445–455
Himmelhoch SR, Karnovsky MJ (1961) Oxidative and hydrolytic enzymes in the nephron of Necturus maculosus: histochemical, biochemical, and electron microscopical studies. J Biophys Biochem Cytol 9:893–908
Hoshi T, Suzuki Y, Itoi K (1981) Differences in functional properties between the early and the late segments of the distal tubule of amphibian (Triturus) kidney. Jpn J Nephrol 23:889–896
Jørgensen PL (1980) Sodium and potassium ion pump in kidney tubules. Physiol Rev 60:864–917
Kaissling B (1980) Ultrastructural organization of the transition from the distal nephron to the collecting duct in the desert rodent Psammomys obesus. Cell Tissue Res 212:475–495
Katz AI (1982) Renal Na−K-ATPase: its role in tubular sodium and potassium transport. Am J Physiol 242:F207-F219
Kawahara K, Sakai T, Hoshi T (1983) The transmural potential of the newt ureter. Evidence for amiloride-sensitive active sodium transport. Jpn J Physiol (in press)
Krause WJ, Cutts JH, Leeson CR (1979) Morphological observations on the mesonephros in the postnatal opossum, Didelphis virginiana. J Anat 129:377–397
Lambert P (1932) Sur les potentialités de résorption du tube contourné chez les urodèles. Compt Rend Soc Biol 110:114–116
Latta H (1973) Ultrastructure of the glomerulus and juxtaglomerular apparatus. In: Orloff J, Berliner RW (ed) Handbook of physiology, section 8: Renal physiology. 1–29, American Physiological Society, Washington
Leeson TS (1959) An electron microscopic study of the mesonephros and metanephros of the rabbit. Am J Anat 105:165–195
Leeson TS (1960) Electron microscopy of the developing kidney: an investigation into the fine structure of the mesonephros and metanephros of the rabbit. J Anat 94:100–106
Linss W (1968) Licht- und elektronenoptische Befunde am Glomerulus der Niere des Hechtes (Esox lucius L). Anat Anz 122:428–448
Linss W, Geyer G (1964) Über die elektronenmikroskopische Struktur der Nierentubul von Rana esculenta. Anat Anz 115:281–296
Long S, Giebisch G (1979) Comparative physiology of renal tubular transport mechanism. Yale J Biol Med 52:525–544
Marshall EK (1934) The comparative physiology of the kidney in relation to theories of renal secretion. Physiol Rev 14:133–189
Maunsbach AB (1973) Ultrastructure of the proximal tubule. In: Orloff J, Berliner RW (ed) Handbook of physiology, section 8: Renal physiology. 31–79, American Physiological Society, Washington
Möllendorff Wv (1930) Der Exkretionsapparat. In: Möllendorff Wv (ed) Handbuch der mikroskopischen Anatomie des Menschen. VII/1, 1–328, Springer, Berlin
Myers CE, Bulger RE, Tisher CC, Trump BF (1966) Human renal ultrastructure. IV. Collecting duct of healthy individuals. Lab Invest 15:1921–1950
Nakamura K, Uéno S-I (1963) Japanese reptiles and amphibians in colour (in Japanese) Hoikusha, Osaka
Nicholson JK (1982) The microanatomy of the distal tubules, collecting tubules and collecting ducts of the starling kidney. J Anat 134:11–23
Osvaldo-Decima L (1973) Ultrastructure of the lower nephron. In: Orloff J, Berliner RW (ed) Handbook of physiology, section 8: Renal physiology. 81–102, American Physiological Society, Washington
Pak Poy RKF (1957a) Electron microscopy of the marsupial renal glomerulus. Austral J Exp Biol 35:437–448
Pak Poy RKF (1957b) Electron microscopy of the amphibian renal glomerulus. Austral J Exp Biol 35:583–594
Pak Poy RKF (1958) Electron microscopy of the piscine (Carassius auratus) renal glomerulus. Austral J Exp Biol 36:191–210
Pak Poy RKF, Robertson JS (1957) Electron microscopy of the avian renal glomerulus. J Biophys Biochem Cytol 3:183–192
Portmann A (1976) Einführung in die vergleichende Morphologie der Wirbeltiere. 5th ed. Schwabe, Basel
Romer AS, Parsons TS (1977) The vertebrate body. 5th ed. Saunders, Philadelphia
Ryan GB, Coghlan JP, Scoggins BA (1979) The granulated peripolar epithelial cell: a potential secretory component of the renal juxtaglomerular complex. Nature 277:655–656
Sakai T, Yohro T (1981) A histological study of the Harderian gland of Mongolian gerbils. Meriones meridianus. Anat Rec 200:259–270
Schiller A, Tiedemann K (1981) The mature mesonephric nephron of the rabbit embryo. III. Freeze-fracture studies. Cell Tissue Res 221:431–442
Schmidt-Nielsen K (1979) Animal physiology: adaptation and environment. 2nd ed. Cambridge University Press, Cambridge
Schønheyder HC, Maunsbach AB (1975) Ultrastructure of a specialized neck region in the rabbit nephron. Kid Int 7:145–153
Solger B (1882) Beiträge zur Kenntnis der Niere und besonders der Nierenpigmente niederer Wirbeltiere. Abhandl Naturf Gesell Halle 15:405–444
Starck D (1982) Vergleichende Anatomie der Wirbeltiere auf evolutionsbiologischer Grundlage. vol 3. Springer, Berlin
Stoner LC (1977) Isolated, perfused amphibian renal tubules; the diluting segment. Am J Physiol 233:F438-F444
Tiedemann K (1979) Architecture of the mesonephric nephron in pig and rabbit. Anat Embryol 157:105–112
Tiedemann K, Wettstein R (1980) The mature mesonephric nephron of the rabbit embryo. I SEM-studies. Cell Tissue Res 209:95–109
Tisher CC, Bulger RE, Trump BF (1968) Human renal ultrastructure. III. The distal tubule in healthy individuals. Lab Invest 18:655–668
Walker AM, Hudson CL (1937) The reabsorption of glucose from the renal tubule in amphibia and the action of phlorhizin upon it. Am J Physiol 118:130–143
Walker AM, Hudson CL, Findley T, Richards AN (1937) The total molecular concentration and the chloride concentration of fluid from different segments of the renal tubule of amphibia. Am J Physiol 118:121–129
Wettstein R, Tiedemann K (1909) The mature mesonephric nephron of rabbit embryo. II. TEM-studies. Cell Tissue Res 218:161–180
Wiedersheim R (1909) Vergleichende Anatomie der Wirbeltiere. 7th ed. Gustav Fischer, Jena
Yamada E (1955) The fine structure of the renal glomerulus of the mouse. J Biophys Biochem Cytol 1:551–566
Yamada E (1960) Collagen fibrils within the renal glomerulus. J Biophys Biochem Cytol 7:407–409
Yamamoto T (1982) Ultrastructural basis of intestinal absorption. Arch Histol Jpn 45:1–22
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakai, T., Kawahara, K. The structure of the kidney of Japanese newts, Triturus (Cynops) pyrrhogaster . Anat Embryol 166, 31–52 (1983). https://doi.org/10.1007/BF00317943
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317943