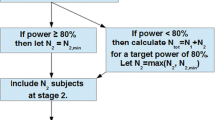
Summary
Guidelines for the performance and analysis of bioequivalence studies are not very specific. The advantages and disadvantages of the following methods and tests are discussed: analysis of variance by summation or by use of general linear models, nonparametric procedures, aposteriori probabilities and tests on the normality of residuals and on the variability of the results. Arguments for or against an analysis of data after logarithmic transformation versus analysis of untransformed data are presented. If the confidence intervals lie within certain limits, preparations may be considered equivalent. The criteria leading to those limits are discussed.
It is recommended that concentration-dependent data of bioequivalence studies be evaluated by analysis of variance after logarithmic transformation, applying general linear models. Data that by theoretical reasons cannot be normally or log-normally distributed should be analysed by nonparametric methods. Otherwise these methods can only be recommended if a significant deviation from normality has been noted and only for two-way cross-over designs. For a geometric evaluation (after logarithmic transformation) the regions of acceptance should be symmetrical in the logarithm, e.g. (80%, 125%).
Similar content being viewed by others
References
Nordic Council on Medicines (1987) Bioavailability studies in man, Nordic guidelines. Nordiska Läkemedelsnämnden, Uppsala (NLN Publ. No 18)
Junginger H (1987) APV-Richtlinie Untersuchungen zur Bioverfügbarkeit, Bioäquivalenz. Dtsch Apoth Z 127: 1645–48, Pharm Ind 49: 704–707
Bioequivalence Task Force (1988) Recommendations from the bioequivalence hearing conducted by the Food and Drug Administration, September 29–October 1, 1986. U.S. Food and Drug Administration, Rockville (Docket No. 86N-025)
Shuirmann DJ (1987) A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 15: 657–680
Scheffé H (1959) The analysis of variance. Wiley and Sons, Chichester Brisbane Toronto Singapore
Grizzle JE (1974) Correction. Biometrics 30: 727
Grizzle JE (1965) The two period change-over design and its use in clinical trials. Biometrics 21: 467–480
Grieve AP (1982) Correspondence. Biometrics 38: 517
Gomez-Marin O, McHugh RB (1983) Analysis of the unbalanced two-period cross-over design with negligible residual effects. Biom J 25: 3–19
Westlake WJ (1973) Use of statistical methods in evaluation of in vivo performance of dosage forms. J Pharm Sci 62: 1579–89
Westlake WJ (1973) The design and analysis of comparative blood level trials. In: Swarbrick J (ed.) Current concepts in the pharmaceutical sciences: Dosage form design and bioavailability. Lea and Febiger, Philadelphia
Steinijans VW, Eicke R, Ahrens J (1982) Pharmacokinetics of theophylline in patients following short-term infusion. Eur J Clin Pharmacol 22: 417–422
Sheiner LB (1985) Analysis of pharmacokinetic data using parametric models. II. Point estimates of an individual's parameters. J Pharmacokinet Biopharm 13: 515–540
Box GEP, Cox DR (1964) An analysis of transformations. J Roy Stat Soc (Ser B) 26: 211–243
Koch GG (1972) The use of nonparametric methods in the statistical analysis of the two-period change-over design. Biometrics 28: 577–584
Pratt JW (1959) Remarks on zeros and ties in the Wilcoxon signed rank procedures. Am Stat Assoc J 54: 655–667
Hollander M, Wolfe DA (1973) Nonparametric statistical methods. Wiley and Sons, New York Chichester Brisbane Toronto Singapore
Flühler H, Grieve AP, Mandallaz D, Mau J, Moser HA (1983): Bayesian approach to bioequivalence assessment: An example. J Pharm Sci 72: 1178–81
Mandallaz D, Mau J (1981) Comparison of different methods for decision-making in bioequivalence assessment. Biometrics 37: 213–222
Anderson S, Hauck WW (1983): A new procedure for testing equivalence in comparative bioavailability and other clinical trials. Commun Statist Theor Meth 12: 2663–92
Hauck WW, Anderson S (1984) A new statistical procedure for testing equivalence in two-group comparative bioavailability trials. J Pharmacokinet Biopharm 12: 83–91
Haynes JD (1983) FDA 75/75 rule: A response. J Pharm Sci 72: 99
Metzler CM (1987) Assessment of variance in bioavailability studies. Comments on the article by McNamara et al. Pharm Res 4: 536
Skelly JP, Shah VP, Shuirmann DJ (1988) Reply to “Assessment of variance in bioavailability studies: Comments on the article by McNamara et al.” by Carl M. Metzler. Pharm Res 5: 322
Haynes JD (1981) Statistical simulation study of new proposed uniformity requirement for bioequivalency studies. J Pharm Sci 70: 673–675
Blume H, Siewert M, Stezhorn G, Kübel-Thiel K (1987) ZL-Monographie zur Prüfung der Bioverfügbarkeit/Bioäquivalenz (Entwurf): Doxycyclin. Dtsch Apoth Z 127: 2090–94, Pharm Z 132: 2476–80
I. Geisler (1988) Bekanntmachung einer Mitteilung der Transparenzkommission an die Hersteller von apothekenpflichtigen Fertigarzneimitteln für kardiavaskuläre Indikationen zur Bioäquivalenz von Nifedipin. Bundesanzeiger 40: 2305–07
Westlake WJ (1976) Symmetrical confidence intervals for bioequivalence trials. Biometrics 32: 741–744
Westlake WJ (1979): Design and statistical evaluation of bioequivalence studies in man. In: Blanchard J, Sawchuk RJ, Brodie BB (eds) Principles and perspectives in drug bioavailability. Karger, Basel, pp 192–210
Lehmacher W, van Eimeren W (1986): Zur statistischen Bewertung der Ergebnisse von Bioverfügbarkeitsstudien. Therapiewoche 36: 413–420
Sachs L (1984) Angewandte Statistik, 6. Aufl. Springer, Berlin Heidelberg New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pabst, G., Jaeger, H. Review of methods and criteria for the evaluation of bioequivalence studies. Eur J Clin Pharmacol 38, 5–10 (1990). https://doi.org/10.1007/BF00314794
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00314794