Summary
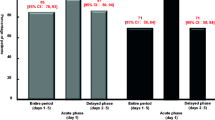
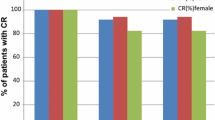
This prospective, randomized, nonblind study comparing the antiemetic effectiveness of high-dose IV metoclopramide and high-dose IV dexamthasone was performed in 78 advanced cancer patients. Chemotherapeutic treatment consisted in cisplatin at a high-dose (120 mg/m2) (HD-CDDP) and at a low-dose (LD-CDDP), either alone (60 mg/m2) or in combination with other chemotherapeutic agents (50 mg/m2). The evaluation of the effectiveness of antiemetic therapy was based on three parameters: prevention of vomiting (“major protection”), number of emetic episodes, and subjective preference. Out of 78 study patients, 67 were evaluable. Overall, metoclopramide proved to be statistically superior to dexamethasone in preventing vomiting (P<0.005), in reducing the median/mean number of emetic episodes (P<0.001/0.001), and in subjective preference (P<0.01). The results divided between HD-CDDP and LD-CDDP groups were also in favor of metoclopramide for reduction of the median/mean number of emetic episodes (P<0.001/0.001 for the HD-CDDP group and P<0.001/0.005 for the LD-CDDP group) and in subjective preference (P<0.001 and P<0.001 for the HD- and LD-CDDP groups, respectively). No statistical differences were noted when LD-CDDP was used in monochemotherapy, whereas when LD-CDDP was used in combination chemotherapy, statistical differences in favor of metoclopramide were noted again for the median/mean number of emetic episodes (P<0.01/0.05) and for subjective preference (P<0.01), even though the effectiveness of both antiemetic agents was greatly reduced. The evaluation of previously untreated patients reflected the overall results: for the HD-CDDP group all three parameters demonstrated statistical significance in favor of metoclopramide; for the LD-CDDP group, of all three parameters, prevention of vomiting (major protection) was the only one for which there was no significant difference.
Mild sedation was the only side effect of metoclopramide. No extrapyramidal reactions were noted during this trial, but concomitant orphenadrine treatment was given. Dexamethasone was always well tolerated. In conclusion, high-dose IV metoclopramide demonstrated its superiority over high-dose IV dexamethasone in all subsets of our population except the LD-CDDP monochemotherapy group, in which the two antiemetics were found to be equivalent in effect.
Similar content being viewed by others
References
Aapro MS, Alberts DS (1981) High-dose dexamethasone for prevention of cisplatin-induced vomiting. Cancer Chemother Pharmacol 7:11–14
Armitage P (1971) Statistical methods in medical research. Blackwell Scientific, Oxford
Bruera E, Roca E, Cedaro L et al (1982) An evaluation of metoclopramide (MCP) and dexamethasone (DM) in the prevention of chemotherapy (CT)-induced emesis. Proc AACR/ASCO 23:55
Bui NB, Marit G, Albin H, et al (1982) Metoclopramide à forte dose au cour de chimiothérapies anticancéreuses lourdes. Etude de phase II chez 80 malades consecutifs. Bull Cancer (Paris) 69:330–335
Buston JL (1981) Methylprednisolone as an antiemetic. N Engl J Med 304:486
Drapkin RL, Sokol GH, Paladine WJ et al (1982) The antiemetic effect and dose response of dexamethasone in patients receiving cis-platinum Proc AACR/ASCO 23:61
Frustaci S, Tirelli U, Tumolo S, et al (1983) High-dose metoclopramide in Cisplatin-treated patients. Chemioterapia II:75–77
Frytac S, Moertel CG (1981) Management of nausea and vomiting in the Cancer patient. JAMA 245:393–396
Gralla RJ, Itri LM, Pisko SE, et al (1981) Antiemetic efficacy of high-dose metochlopramide: randomized trials with placebo and chlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J Med 35:905–909
Gralla RJ, Tyson LB, Clark RA, et al (1982) Antiemetic trials with high-dose metoclopramide: superiority over THC, and preservation of efficacy in subsequent chemotherapy courses. Proc AACR/ASCO 23:58
Herman TS, Einhorn LH, Jones SE, et al (1979) Superiority of nabilone over prochlorperazine as an antiemetic in patients receiving cancer chemotherapy. N Engl J Med 300:1295–1297
Lazlo J, Hanson DC, Lucas VS, Clark R, Tyson L, Gralla R, Derivan A (1983) Lorezepan as an antiemetic against cisplatin. Proc Am Soc Clin Oncol 2:95
Long A, Mioduszewski J, Natale R (1982) A randomized double-blind cross-over comparison of the antiemetic activity of levonantradol and prochlorperazine. Proc AACR/ASCO 23:57
Mason BA, Dambra J, Grossman B et al (1982) Effective control of Cis-platin-induced nausea using high-dose steroids and droperidol. Cancer Treat Rep 66:243–245
Meyer M, Long AM, Natale RB, Koller C, Mioduszewski J (1983) Phase I, II and III trials of new antiemetic agentlorazepam. Proc Am Soc Clin Oncol 2:88
Prestayko AW, D'Aoust JC, Issel BF, et al (1979) Cisplatin (cisdiamminedicloroplatinum) (II). Cancer Treat Rev 6:17–39
Rich WM, Abdulhayogen G, Di Saia PJ (1980) Methylprednisolone as an antiemetic during cancer chemotherapy—a pilot study. Gynecol Oncol 9:193–198
Sallan SE, Cronin CM (1982) Nausea and vomiting. In: De Vita VT, Hellman S, Rosenberg SA. Cancer principles and practice of oncology (1st edn) Lippincott, Philadelphia, pp 1704–1707
Sallan SE, Zinberg NE, Nelson JM (1975) Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. New Engl J Med 293:795–797
Seigel LJ, Longo DL (1981) The control of chemotherapy-induced emesis. Ann Intern Med 95:352–359
Siegel S (1956) Nonparametric statistics for the behavioral sciences. McGraw-Hill, New York
Stambough JE, McAdams J, Vreeland F (1982) A phase II randomized trial of the antiemetic activity of Levonantradol (CP-50,556) in cancer patients receiving chemotherapy. Proc AACR/ASCO 23:62
Steel N, Gralla RJ, Braun DW et al (1980) Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on themotherapy-induced emesis. Cancer Treat Rep 64:219–214
Von Hoff DD, Shilsky R, Reichest CM, et al (1979) Toxic effects of Cisdichlorodiammineplatinum (II) in man. Cancer Treat Rep 63:1527–1531
Williams CJ, Bolton A, De Pemberton R, et al (1980) Antiemetics for patients treated with antitumor chemotherapy. Cancer CI Trials 3:363–367
Author information
Authors and Affiliations
Additional information
Oral presentation at the 19th annual meeting of the American Society of Clinical Oncology, San Diego, CA, May 22–24, 1983
Rights and permissions
About this article
Cite this article
Frustaci, S., Grattoni, E., Tumolo, S. et al. Randomized crossover antiemetic study in cisplatin-treated patients. Cancer Chemother. Pharmacol. 17, 75–79 (1986). https://doi.org/10.1007/BF00299870
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00299870