Abstract
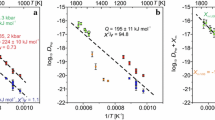
We present new experimental data on diffusion of divalent cations in almandine-spessartine diffusion couples in graphite capsules in the P-T range of 14–35 kb, 1100–1200° C. The tracer diffusion coefficients of the major divalent cations, viz. Fe, Mg and Mn, retrieved from the multicomponent diffusion profiles, have been combined with earlier data from our laboratory at 29–43 kb, 1300–1480° C (Loomis et al. 1985) to derive expressions of the P-T dependence of the diffusion coefficients at fO2 approximately corresponding to that defined by equilibrium in the system graphite-O2. We review the conditions, discussed earlier by Cooper, under which the flux of a component in a multicomponent system becomes proportional to its concentration gradient (Fickian diffusion), as if the entire solvent matrix behaves as a single component, and also suggest a method of incorporating the thermodynamic effect on diffusion in the same spirit. Regardless of the magnitude or sign of the off-diagonal terms of the D matrix, it is always possible to define an effective binary diffusion coefficient (EBDC) of a component in a semi-infinite multicomponent diffusion-couple experiment such that it has the property of the Fickian diffusion coefficient, provided that there is no inflection on the diffusion profiles. It is shown that the success of Elphick et al. in fitting the experimental diffusion profiles of all components over a limited concentration range by a single diffusion coefficient is due to fortuitous similarity of the EBDCs of the components (Fe, Mg, Mn and Ca) in their diffusion couple experiments. In common metapelitic garnets showing compositional zoning, the EBDCs of the divalent cations do not differ from each other by more than a factor of 2.5. However, the EBDC of a component changes from core to rim by a factor of 3 to 12, depending on the composition. We suggest a method of volume averaging of the EBDC which should prove useful in approximate calculations of diffusion flux during relaxation of compositional zoning. The EBDC of Mn is found to reduce essentially to D MnMn, the main diagonal term of the D matrix, and consequently can be calculated quite easily. Evaluation of EBDC of Fe, Mg and Mn in garnets from a prograde Barrovian sequence did not reveal any significant dependence on the extent of relaxation of garnet. The diffusion data have been applied to calculate the cooling rate of natural biotite-garnet diffusion couple from eastern Finland and diffusional modification of growth zoning in garnet in early Proterozoic Wopmay orogen, Canada. The results are in good agreement with geochronological and other independent constraints.
Similar content being viewed by others
Abbreviations
- a :
-
Radius of a spherical garnet crystal
- BSE :
-
Back-scattered electron imaging
- C :
-
Column vector of (n-1) independent components
- D :
-
Diffusion coefficient matrix
- D ij :
-
An element of the diffusion matrix on the i th row and j th column
- D i* :
-
Tracer diffusion coefficient of component i
- D(i) :
-
Effective interdiffusion coefficient (EIC) of various components in a multicomponent solution rich in the component i
- D(i-j) :
-
Interdiffusion coefficient of components i and j in a binary solution
- D i (EB):
-
Effective binary diffusion coefficient of component i in a multicomponent solution
- D i (EB:Ideal):
-
D i (EB) under condition of ideal thermodynamic mixing of the diffusing species
- D i (EB:thermo):
-
Thermodynamic component of D i(EB)
- D AO :
-
Interdiffusion coefficient at peak temperature T 0 in the phase A
- D 0 :
-
Pre-exponential factor in an Arrhenius relation
- EBDC :
-
Effective binary diffusion coefficient between a solute and a multicomponent solvent matrix
- FEC :
-
Fixed edge composition model
- EIC :
-
Effective interdiffusion coefficient
- f i :
-
Fugacity of component i
- HM :
-
Hematite-magnetite oxygen fugacity buffer
- kb:
-
Kilobars
- P :
-
Pressure
- Q :
-
Activation energy (enthalpy) of diffusion
- \(\mathbb{R}\) :
-
Extent of relaxation defined as the difference between core and rim compositions normalized to the same difference in the initial zoning profile
- R :
-
Gas constant
- s :
-
Cooling rate
- T 0, T Ch :
-
Peak temperature and characteristic temperature, respectively
- t :
-
Time
- VEC :
-
Variable edge composition model
- ΔV + :
-
Activation volume
- W ij :
-
Simple mixture interaction parameter between i and j
- W i(EB):
-
Effective simple mixture interaction parameter of a component i in a multicomponent solution
- Ŵ ij :
-
Margules interaction parameter between i and j
- X i :
-
Mole fraction of component i
- τi :
-
Activity coefficient of component i
- ϕ:
-
A dimensionless variable =πD t/a 2
- δ ij :
-
Kronecker delta (i=j, δ ij =1; i≠j, δ ij =0)
- Zi :
-
Charge on the ion i
References
Akella J, Vaidya SN, Kennedy GC (1969) Melting of sodium chloride at pressures to 65 kbar. Physical Rev 185:1135–1140
Anovitz LMA, Chase CG (1990) Implications of post-thrusting extension and underplating for P-T-t paths in granulite terranes: a Grenville example. Geology 18:466–469
Barrer RM, Bartholomew RF, Rees LVC (1963) Ion exchange in porous crystals. Part II. The relationship between self and exchange-diffusion coefficients. J Phys Chem Solids 24:309–317
Boettcher AL, Windom KE, Bohlen SR, Luth RW (1981) Low friction, anhydrous, low-to high-temperature furnace assembly for piston cylinder apparatus. Rev Sci Instrum 52:1903–1904
Brady JB (1975) Reference frames and diffusion coefficients. Am J Sci 275:954–983
Chakraborty S, Ganguly J (1990) Compositional zoning and cation diffusion in aluminosilicate garnets. In: Ganguly J (ed) Diffusion, atomic ordering and mass transfer, advances in physical geochemistry, Vol 8. Springer, Berlin Heidelberg New York Tokyo, pp 120–175
Clark SP Jr (1959) Effect of presure on the melting point of eight alkali halides. J Chem Phys 31:1526–1531
Cohen LH, Klement W Jr, Kennedy GC (1966) Investigation of phase transitions at elevated temperatures and pressures by differential thermal analysis in pistoncylinder apparatus. J Phys Chem Solids 27:179–186
Cooper AR (1968) The use and limitation of the concept of an effective binary diffusion coefficient for multicomponent diffusion, in Mass Transport in Oxides-Proc Symposium, US Dept of Commerce, NBS Sp Pub No 296
Crank J (1975) The mathematics of diffusion, Oxford London, 414 pp
Cullinan HT Jr (1965) Analysis of the flux equations of multicomponent diffusion. Ind Eng Chem Fundament 4:133–139
Cygan RT, Lasaga AC (1985) Self diffusion of magnesium in garnet at 750° C to 900° C. Am J Sci 285:328–350
Darken LS (1948) Diffusion, mobility and their interrelation through free energy in binary metallic systems. Am Inst Mining Metall Engineers Trans 175:184–201
Dempster TJ (1985) Garnet zoning and metamorphism of the Barrovian type area, Scotland. Contrib Mineral Petrol 89:30–38
Elphick SC, Ganguly J, Loomis TP (1981) Experimental study of Fe−Mg interdiffusion in aluminosilicate garnet. EOS (abstract). Tran Am Geophys Union 62:411
Elphick SC, Ganguly J, Loomis TP (1985) Experimental determination of cation diffusivities in aluminosilicate garnets I. Experimental methods and interdiffusion data. Contrib Mineral Petrol 90:36–44
England PC, Thompson AB (1984) Pressure-temperature-time paths of metamorphism I. Heat transfer during the evolution of regions of thickened continental crust. J Petrol 25:894–928
Ferry JM, Spear FS (1978) Experimental calibration of the partitioning of Fe and Mg between biotite and garnet. Contrib Mineral Petrol 66:113–117
Florence FP, Spear FS (1991) Effects of diffusional modification of garnet growth zoning on P-T path calculations. Contrib Mineral Petrol 107:487–500
Freer R (1981) Diffusion in silicate minerals and glasses: a data digest and guide to literature. Contrib Mineral Petrol 76:440–454
Ganguly J, Kennedy GC (1974) The energetics of natural garnet solid solution. I. Mixing of the aluminosilicate end-members. Contrib Mineral Petrol 48:137–148
Ganguly J, Bhattacharya RN, Chakraborty S (1988) Convolution effect in the determination of compositional profiles and diffusion coefficients by microprobe step scans. Am Mineral 73:901–909
Ganguly J, Saxena SK (1984) Mixing properties of aluminosilicate garnets: Constraints from natural and experimental data, and applications to geothermobarometry. Am Mineral 69:88–97
Ganguly J, Ruiz J (1987) Time-temperature relation of mineral isochrons: a thermodynamic model, and illustrative applications for the Rb−Sr system. Earth Planet Sci Let 81:338–348
Ganguly J, Saxena SK (1987) Mixtures and mineral reactions. Springer, Berlin Heidelberg New York Tokyo
Ganguly J, Chakraborty S, Rumble D III (1991) Constraint on the time scale of regional metamorphism from diffusion profiles in a natural garnet-garnet couple from Vermont. EOS (abstract) Am Geophys Union, Fall meeting
Geiger CA, Newton RC, Kleppa OJ (1987) Enthalpy of mixing of synthetic almandine-grossular and almandine-pyrope garnets from high temperature solution calorimetry. Geochim Cosmochim Acta 51:1755–1763
Guggenheim EA (1967) Thermodynamics. Elsevier, North Holland Amsterdam New York
Hackler RT, Wood BJ (1989) Experimental determination of Fe and Mg exchange between garnet and olivine and estimation of Fe−Mg garnet mixing properties. Am Mineral 74:994–999
Haselton HT, Newton RC (1980) Thermodynamics of pyrope-grossular garnets and their stabilities at high temperatures and pressures. J Geophys Res 85:6973–6982
Hodges KV, Spear FS (1982) Geothermometry, geobarometry and the Al2SiO5 triple point at Mt. Moosilauke, New Hampshire. Am Mineral 67:1118–1134
Hollister LS (1969) Contact metamorphism in the Kwoiek Area of British Columbia: An end member of the metamorphic process; Geol Soc Am Bull 80:2465–2494
Jiang J, Lasaga AC (1990) The effect of post-growth thermal events on growth-zoned garnet: Implications for metamorphic P-T history calculations. Contrib Mineral Petrol 105:454–459
Koziol AM (1990) Activity-composition relationships of binary Ca−Fe and Ca−Mn garnets determined by reversed, displaced equilibrium experiments. Am Mineral 75:319–327
Koziol AM, Newton RC (1989) Grossular activity-composition relationships in ternary garnets determined by reversed displaced equilibrium experiments. Contrib Mineral Petrol 103:423–433
Lasaga AC (1979) Multicomponent exchange and diffusion in silicates. Geochim Cosmochim Acta 43:455–469
Lasaga AC (1983) Geospeedometry: An extension of geothermometry. In: Saxena SK (ed) Kinetics and equilibrium in mineral reactions, advances in physical geochemistry, Vol 3. Springer, Berlin Heidelberg New York Tokyo, pp 81–114
Lasaga AC, Richardson SM, Holland HD (1977) The mathematics of cation diffusion and exchange between silicate minerals during retrograde metamorphism. In: Saxena SK, Bhattacharji SD (ed) Energetics of geologic processes. Springer, BAR, Berlin Heidelberg New York Tokyo, pp 353–388
Lindstrom R, Vitanen M, Juhanoja J, Holtta P (1991) Geospeedometry of metamorphic rocks: examples in the Rantasalmi-Sulkava and Kiuruvesi areas, eastern Finland. Biotite-garnet diffusion couples. J Metamorphic Geol 9:181–190
Loomis TP (1986) Metamorphism in metapelites: calculation of equilibrium assemblages and numerical simulations of the crystallization of garnet. J Met Geol 4:201–230
Loomis TP, Ganguly J, Elphick SC (1985) Experimental determination of cation diffusivities in aluminosilicate garnets II. Multicomponent simulation and tracer diffusion coefficients. Contrib Mineral Petrol 90:45–51
Luth R, Virgo D, Boyd FR, Wood BJ (1991) Ferric iron in mantle derived garnets: implications for thermobarometry and for the oxidation state of the mantle. Contrib Mineral Petrol 104:56–72
Manning JR (1968) Diffusion kinetics for atoms in crystals. Van Nostrand, Princeton, USA
McLellan E (1985) Metamorphic reactions in the kyanite and sillimanite zones of the Barrovian type area. J Petrol 26:789–818
Morioka M (1981) Cation diffusion in olivine-II. Ni−Mg, Mn−Mg, Mg and Ca. Geochim Cosmochim Acta 45:1573–1580
Morioka M, Nagasawa H (1990) Ionic diffusion in olivine. In: Ganguly J (ed) Diffusion, atomic ordering and mass transport, advances in physical geochemistry, Vol 8. Springer, Berlin, Heidelberg, New York, Tokyo, pp 176–197
Muncill GE, Chamberlain CP (1988) Crustal cooling rates inferred from homogenization of metamorphic garnets. Earth Planet Sci Lett 87:390–396
Onsager L (1945) Theories and problems of liquid diffusion. New York Acad Sci Ann 46:241–265
Perchuk LL, Lavrent'eva IV (1983) Experimental investigation of exchange equilibria in the system cordierite-garnet-biotite. In: Saxena SK (ed) Kinetics and equilibrium in mineral reactions, advances in physical geochemistry Vol 3. Springer, Berlin Heidelberg New York Tokyo, pp 199–239
Powenceby MI, Wall VJ, O'Neill HStC (1987) Fe−Mn partitioning between garnet and ilmenite: experimental calibration and applications. Contrib Mineral Petrol 97:116–126
Press WH, Flannery BP, Teukolsky SA, Vetterling WT (1986) Numerical recipes — The art of scientific computing. Cambridge University Press, Cambridge, UK
Robie RA, Hemingway BS, Fisher JR (1978) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperature. US Geol Survey Bull Vol 1452. Washington, USA
Saxena SK, Fei Y (1987) Fluids at crustal pressures and temperatures I. Pure species. Contrib Mineral Petrol 95:370–375
Shewmon PG (1963) Diffusion in solids. McGraw-Hill, New York, USA
Smith D, Barron BR (1991) Pyroxene-garnet equilibration during cooling in the mantle. Am Mineral (in press)
Spear FS, Silverstone J (1983) Quantitative P-Tpaths from zoned minerals: theory and tectonic applications. Contrib Mineral Petrol 83:348–357
St-Onge MR (1987) Zoned poikiloblastic garnets: P-Tpaths and synmetamorphic uplift through 30 km of structural depth, Wopmay Orogen. Canada, J Petrol 28:1–21
Tompson AFB, Gray WG (1986) A Second Order Approach for the modeling of Dispersive Transport in Porous Media I. Theoretical Development, Water Resources Res 22:591–599
Toor HL (1964) Solution of the Linearized equations of Multicomponent mass transfer: II. Matrix Methods. J Am Inst Chem Eng 8:460–465
Tracy RJ, Robinson P, Thompson AB (1976) Garnet composition and zoning in the determination of temperature and pressure of metamorphism, central Massachusetts. Am Mineral 61:762–775
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chakraborty, S., Ganguly, J. Cation diffusion in aluminosilicate garnets: experimental determination in spessartine-almandine diffusion couples, evaluation of effective binary diffusion coefficients, and applications. Contrib Mineral Petrol 111, 74–86 (1992). https://doi.org/10.1007/BF00296579
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00296579