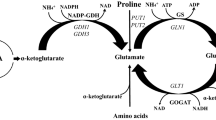
Summary
Mutants lacking NADP-linked glutamate dehydrogenase (NADP-GDH) activity have been isolated by several procedures.
Complementation tests in diploids as well as tetrad analysis show that they map within a short chromosome segment, the gdhA locus, which is allelic to the ure1 locus described previously.
That the gdhA locus is a structural gene for NADP-GDH is supported by two kinds of evidence. First, intracistronic complementation, as well as negative complementation were observed between some of the gdhA- mutants. This is in agreement with the multimeric structure of the NADP-GDH in Saccharomyces cerevisiae shown by Venard and Fourcade (1972). Secondly, some of the mutants at the gdhA locus have a NADP-GDH with modified properties, including: five-fold higher Km for 2-oxoglutarate, hundred-fold higher Km for NH +4 , loss of inhibition by excess of substrate (2-oxoglutarate), and lower thermostability.
Mutants with derepressed NAD-GDH activity have been isolated from gdhA- strains on the basis of their faster growth on ammonia as sole nitrogen source. They define the gdhCR locus, which is allelic to ure2 and usu described previously. This is a strong indication that residual growth of the gdhA- mutants on ammonia as sole nitrogen source is due to the NAD-GDH activity.
Similar content being viewed by others
References
Ahmed, S. I., Sanwal, B. D.: A structural gene for the DPN-specific glutamate dehydrogenase in Neurospora. Genetics 55, 359–364 (1967)
Arst, H. N., McDonald, D. W.: A mutant of Aspergillus nidulans lacking NADP-linked glutamate dehydrogenase. Molec. gen. Genet. 122, 261–265 (1973)
Boehringer, C. F.: Biochimica Catalogue. Mannheim 1968
Drillien, R., Aigle, M., Lacroute, F.: Yeast mutants pleiotropically impaired in the regulation of the two glutamate dehydrogenases. Biochem. biophys. Res. Commun. 53, 367–372 (1973)
Drillien, R., Lacroute, F.: Ureidosuccinec-acid uptake in yeast and some aspects of its regulation. J. Bact. 109, 203–208 (1972)
Dubois, E., Grenson, M., Wiame, J.-M.: Release of the “ammonia effect” on three catabolic enzymes by NADP-specific glutamate dehydrogenaseless mutations in Saccharomyces cerevisiae. Biochem. biophys. Res. Commun. 50, 967–972 (1973)
Elmerich, C.: Le cycle du glutamate, point de départ du métabolisme de l'azote, chez Bacillus megaterium. Europ. J. Biochem. 27, 216–224 (1972)
Elmerich, C., Aubert, J.-P.: Synthesis of glutamate by a glutamine: 2 oxo-glutarate aminotransferase (NADP oxidoreductase) in Bacillus megaterium. Biochem. biophys. Res. Commun. 42, 371–376 (1971)
Fincham, J. R. S.: Mutant strains of Neurospora deficient in aminating ability. J. biol. Chem. 182, 61–73 (1950)
Fincham, J. R. S.: Genetic complementation. New York, Amst.: W. A. Benjamin Inc. 1966
Grenson, M.: The utilization of exogenous pyrimidines and the recycling of uridine-5′-phosphate derivatives in Saccharomyces cerevisiae as studied by means of mutants affected in pyrimidine uptake and metabolism. Europ. J. Biochem. 11, 249–260 (1969)
Grenson, M., Hou, C.: Ammonia inhibition of the general amino acid permease and its suppression in NADPH-specific glutamate dehydrogenaseless mutants of Saccharomyces cerevisiae. Biochem. biophys. Res. Commun. 48, 749–756 (1972)
Grenson, M., Hou, C., Crabeel, M.: Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid nermease. J. Bact. 103, 770–777 (1970)
Grenson, M., Mousset, M., Wiame, J.-M., Bechet, J.: Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim. biophys. Acta (Amst.) 127, 325–338 (1966)
Grenson, M., Ramos, F., Wiame, J.-M.: A glutamic dehydrogenaseless (NADP) mutant in Saccharomyces cerevisiae. In: Handbook of biochemistry, selected data for molecular biology, 2nd ed., p. 83. The chemical Rubber Co., Cleveland, Ohio: H. A. Sober 1970
Holzer, H., Hierholzer, G., Witt, I.: The role of glutamate dehydrogenases in the linkage and regulation of carbohydrate and nitrogen metabolism in Yeast. Colloques internationaux du C.N.R.S. (Paris) 124, 407–416 (1965)
Holzer, H., Schneider, S.: Anreicherung und Trennung einer DPN-spezifischen und einer TPN-spezifischen Glutaminsäure-dehydrogenase aus Hefe. Biochem. Z. 329, 361–369 (1957)
Lacroute, F.: Régulation de la chaîne de biosynthese de l'uracile chez Sacchromyces cerevisiae. Thèse, Paris (1966)
Mortimer, R. K., Hawthorne, D. C.: Genetic mapping in Saccharomyces. Genetics 53, 165–173 (1966)
Rytka, J.: Lack of general amino acid permease activity as a cause of D-amino acid resistance in mutants of Saccharomyces cerevisiae. Proceedings of the Third International Specialized Symposium on Yeasts (H. Suomalainen, C. Waller, eds.), part I, p. 116. Print Oy, Helsinki, 1973
Sanwal, B. D., Lata, M.: Occurrence of two different glutamic dehydrogenases in Neurospora. Canad. J. Microbiol. 7, 319–328 (1961)
Stachow, C. S., Sanwal, B. D.: Regulation, purification and some properties of the NAD-specific glutamate dehydrogenase of Neurospora. Biochim. biophys. Acta (Amst.) 139, 294–307 (1967)
Tempest, D. W., Meers, J. L., Brown, C. M.: Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem. J. 117, 405–407 (1970)
Tempest, D. W., Meers, J. L., Brown, C. M.: Glutamate synthetase (GOGAT): a key enzyme in the assimilation of ammonia by prokaryotic organisms In: The enzymes of glutamine metabolism (S. Prusiner and E. R. Stadtman, eds.) New York: Academic Press p. 167–182, 1973
Venard, R., Fourcade, A.: Glutamate déshydrogénase de levure spécifique de NADP. II. Quelques paramètres moléculaires. Biochimie 54, 1381–1389 (1972)
Author information
Authors and Affiliations
Additional information
Communicated by W. Gajewski
Aspirant du Fonds National de la Recherche Scientifique Belge.
Rights and permissions
About this article
Cite this article
Grenson, M., Dubois, E., Piotrowska, M. et al. Ammonia assimilation in Saccharomyces cerevisiae as mediated by the two glutamate dehydrogenases. Molec. Gen. Genet. 128, 73–85 (1974). https://doi.org/10.1007/BF00267295
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00267295