Summary
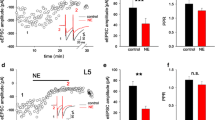
The effects of iontophoretic application of 1-norepinephrine (NE) and related drugs on granule cell responses evoked by a stimulus pulse applied to the medial perforant pathway were studied in anesthetized rats. Drugs were applied and responses recorded at successive dorso-ventral positions along the dendrites and at the cell body layer. 1. Reciprocal actions of alpha and beta receptors were revealed in the cell body region. The beta agonist isoproterenol decreased the population spike while the beta antagonist sotalol increased it. In contrast, the alpha agonists phenylephrine and clonidine increased the population spike whereas the alpha antagonist prazosin decreased it. The action of the drugs was rapid, dose dependent and reversible. NE itself had no effect when applied in the granule cell layer. 2. In contrast to the failure of NE to elicit a short term response, and in confirmation of a previous report (Neuman and Harley 1983), the prolonged application of NE in the granule cell layer produced a longterm enhancement of the population spike. However, this effect was also observed after the application of d-NE. 3. NE affected granule cell responses in the middle third of the dendrites where it reduced the evoked synaptic potential (ESP, current flow produced by excitatory synaptic activity) in a dose-dependent manner. Recordings taken simultaneously in the cell body region revealed a reduction of the population spike and no change in the ESP at the cell body layer (the positive-going ESP reflecting an outward current flow from the cell). In an attempt to delineate receptor specificity, a series of alpha and beta agonists and antagonists were applied to the mid-dendritic region. All drugs reduced the ESP in a manner similar to NE. Such lack of specificity in the action of NE has been previously reported in the spinal motoneuron (Engberg et al. 1976; Marshall 1983). The function of NE in the dentate gyrus is discussed in the light of these and previous results.
Similar content being viewed by others
References
Amaral DG (1978) A Golgi study of cell types in the hilar layer of the hippocampus in the rat. J Comp Neurol 182: 851–914
Andersen P (1983) Operational principles of hippocampal neurons. In: Seifert W (ed) Neurobiology of the hippocampus. Academic Press, New York, pp 84–85
Armstrong-James M, Fox K (1983) Effects of iontophoresis of noradrenalin on the spontaneous activity of neurons in rat primary somatosensory cortex. J Physiol (Lond) 335: 427–447
Aston-Jones G, Bloom FE (1981) Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1: 887–900
Azmitia EC, Segal M (1978) An autoradiographic analysis of the differential ascending projection of the dorsal and median rephe nuclei in the rat. J Comp Neurol 179: 641–668
Berthelsen S, Pettinger WA (1977) A functional fasis for classification of alpha-adrenergic receptors. Life Sci 21: 595–606
Dahl D, Bailey WH, Winson J (1983) Effect of norepinephrine depletion of hippocampus con neuronal transmission from perforant pathway through dentate gyrus. J Neurophysiol 49: 123–133
Dahl D, Winson J (1985) Action of norepinephrine in the dentate gyrus. I. Stimulation of locus coeruleus. Exp Brain Res 59: 491–496
Engberg I, Flatman JA, Kadzielawa K (1976) Lack of specificity of motoneuron responses to microiontophoretically applied phenolic amines. Acta Physiol Scand 96: 137–139
Haas HL (1983) Amine neurotransmitter actions in the hippocampus. In: Seifert W (ed) Neurobiology of the hippocampus. Academic Press, New York, p 147
Haas HL, Konnerith R (1983) Histamine and noradrenaline decrease calcium activated potassium conductance in hippocampal pyramidal cells. Nature 302: 432–434
Jones BE, Moore RY (1977) Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 127: 23–53
Koda LA, Bloom FE (1977) Light and electron microscopic study of noradrenergic terminals in the rat dentate gyrus. Brain Res 120: 327–335
Koehler C, Haglund L, Swanson L (1984) A diffuse alpha MSH immunoreactive projection to the hippocampus and spinal cord from individual neurons in the lateral hypothalamic area and zona incerta. J Comp Neurol 223: 501–514
Lacaille JC, Harley CW (1983) In vitro superfusion of norepinephrine potentiates the perforant evoked field potential in the dentate gyrus. Soc Neurosci Abstr 9: 1001
Langmoen IA, Segal M, Andersen P (1981) Mechanisms of norepinephrine action on hippocampal pyramidal cells in vitro. Brain Res 208: 349–362
Lomo T (1971) Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res 12: 18–45
Loy R, Koziell DA, Lindsey JD, Moore RY (1980) Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol 189: 699–710
Madison DV, Nicoll RA (1982) Noradrenalin blocks the accomodation of pyramidal cell discharge in the hippocampus. Nature 299: 636–638
Marshall KC (1983) Catecholamines and their actions in the spinal cord. In: Davidoff RA (ed) Handbook of the spinal cord. Marcel Dekker, Inc., New York, pp 275–328
Marshall KC, Engberg I (1979) Reversal potential for noradrenalin-induced hyperpolarization of spinal motoneurons. Science 205: 422–424
Moore RY, Hilaris AE (1975) Hippocampal innervation by serotonin neurons of the midbrain raphe in the rat. J Comp Neurol 164: 171–184
Mueller AL, Hoffer BJ, Dunwiddie TV (1981) Noradrenergic responses in the rat hippocampus: evidence for mediation by alpha and beta receptors in the in vitro slice. Brain Res 214: 113–126
Mueller AL, Palmer MR, Hoffer BJ, Dunwiddie TV (1982a) Hippocampal noradrenergic responses in vivo and in vitro. Naun-Schmiedeberg's Arch Pharmacol 318: 259–266
Mueller AL, Kirk HL, Hoffer PJ, Dunwiddie TV (1982b) Noradrenergic responses in rat hippocampus: electrophysiological actions of direct- and indirect-acting sympathomimetics in the in vitro slice. J Pharmacol Exp Ther 223: 599–605
Neuman RS, Harley CW (1983) Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res 273: 162–165
Palacios JM, Kuhar MJ (1982) Beta adrenergic receptor localization in rat brain by light microscopic autoradiography. Neurochem Internat 4: 473–490
Rogawski MA, Aghajanian GK (1980) Activation of lateral geniculate neurons by norepinephrine: mediation by an alpha-adrenergic receptor. Brain Res 182: 345–359
Rainbow TC, Parsons B, Wolfe BB (1984) Quantitative autoradiography of beta1- and beta2-adrenergic receptors in rat brain. Proc Nat Acad Sci 81: 1585–1589
Swanson LW, Cowan WM (1979) The connections of the septal region in the rat. J Comp Neurol 186: 621–656
Swanson LW, Hartman BK (1975) The central adrenergic system. An immunofluorescence study of the localization of cell bodies and their efferent connections in the rat utilizing beta-hydroxylase as a marker. J Comp Neurol 163: 467–506
Szabadi E (1979) Adrenoceptors on central neurones: microelectrode studies. Neuropharmacology 18: 831–843
Winson J (1980) Influence of raphe nuclei on neuronal transmission from perforant pathway through dentate gyrus. J Neurophysiol 44: 937–950
Winson J, Abzug C (1978) Neuronal transmission through hippocampal pathways dependent on behavior. J Neurophysiol 41: 716–732
Wyss JN, Swanson LW, Cowan WN (1979) Evidence for an input to the molecular layer and the stratum granulosum of the dentate gyrus from the supramammilary region of the hypothalamus. Anat Embryol 156: 165–176
Young III WS, Kuhar NJ (1980) Noradrenergic alpha1 and alpha2 receptors: light microscopic autoradiographic localization. Proc Nat Acad Sci 77: 1696–1700
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Winson, J., Dahl, D. Action of norepinephrine in the dentate gyrus. II. Iontophoretic studies. Exp Brain Res 59, 497–506 (1985). https://doi.org/10.1007/BF00261340
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00261340