Summary
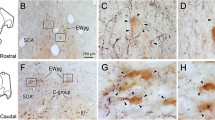
This study documents a bilateral projection from nucleus reticularis tegmenti pontis (NRTP) to the rostral aspect of the medial vestibular nucleus (MVN) in rabbits. Horseradish peroxidase injections in rostral MVN produced retrogradely labeled neurons in the caudal half of NRTP; caudal MVN injections produced negative results. This supports the hypothesis that NRTP relays visual input to the vestibular nuclei via an extracerebellar pathway (Precht and Strata 1980), and indicates the importance of examining the contributions of both direct and cerebellar-mediated visual pathways to oculomotor physiology.
Similar content being viewed by others
References
Balaban CD (1983) Olivo-vestibular and cerebellovestibular connections in albino rabbits: Relationship to vestibulocerebellar microzones. Neuroscience (in press)
Balaban CD, Kawaguchi Y, Watanabe E (1981) Evidence of a collateralized climbing fiber projection from the inferior olive to the flocculus and vestibular nuclei in rabbits. Neurosci Lett 22: 23–29
Batton RR, Jayaraman A, Ruggiero D, Carpenter MB (1979) Fastigial efferent projections in the monkey: an autoradio-graphic study. J Comp Neurol 174: 281–306
Bechterew W (1885) Zur Anatomie der Schenkel des Kleinhirns insbesondere der Bruckenarne. Neurol Centralbl 4: 121–125
Brodal A, Brodal P (1971) The organization of the nucleus reticularis tegmenti pontis in the cat in light of experimental anatomical studies of its cerebral cortical afferents. Exp Brain Res 13: 90–110
Brodal A, Lacerda AM, Destombes J, Angaut P (1972) The pattern of projection of the intracerebellar nuclei onto the nucleus reticularis tegmenti pontis in the cat. An experimental anatomical study. Exp Brain Res 16: 140–160
Brodal P (1980) The cortical projection to the nucleus reticularis tegmenti pontis in the rhesus monkey. Exp Brain Res 38: 19–27
Fukuda J, Highstein SM Ito M (1972) Cerebellar inhibitory control of the vestibulo-ocular reflex investigated in the rabbit IIIrd nucleus. Exp Brain Res 14: 511–526
Graham RC, Karnovsky MJ (1967) The early stages of absorption of injected horseradish peroxidase in proximal tubules of mouse kidney, Ultrastruct Cytochem 14: 291–302
Graybiel A, Nauta HJW, Lasek RJ, Nauta WJH (1973) A cerebello-olivary pathway in the cat: an experimental study using autoradiographic tracing techniques. Brain Res 58: 205–211
Haddad GM, Demer JL, Robinson DA (1980) The effect of lesions of the dorsal cap of the inferior olive in the cat. Brain Res 185: 265–275
Hoddevik GH, Brodal A, Walberg F (1975) The reticulo-vestibular projection in the cat. An experimental study with silver impregnation methods. Brain Res 94: 383–399
Ito M, Miyashito Y (1975) The effects of chronic destruction of the inferior olive upon visual modification of the horizontal vestibulo-ocular reflex of rabbits. Proc Jpn Acad 51: 716–720
Keller EL, Precht W (1978) Persistence of visual response in vestibular nucleus neurons in cerebellectomized cat. Neurosci Lett 32: 591–594
Keller EL, Precht W (1979) Visual-vestibular responses in vestibular nuclear neurons in the intact and cerebellectomized, alert cat. Neuroscience 4: 1599–1613
Kitai ST, Kocsis JD, Kiyohara T (1976) Electrophysiological properties of nucleus reticularis tegmenti pontis cells: antidromic and synaptic activation. Exp Brain Res 24: 295–309
Kooy FH (1917) The inferior olive in vertebrates. Folia Neurobiol 10: 205–369
Ladpli R, Brodal A (1968) Experimental studies of commisural and reticular formation projections from the vestibular nuclei in the cat. Brain Res 8: 65–96
Maekawa K, Takeda T (1975) Mossy fiber responses evoked in the cerebellar flocculus of rabbits by stimulation of the optic pathway. Brain Res 98: 590–595
Maekawa K, Takeda T (1976) Electrophysiological identification of the climbing and mossy fiber pathways from the rabbit's retina to the contralateral cerebellar flocculus. Brain Res 109: 169–174
Maekawa K, Takeda T (1978) Origin of the mossy fiber projection to the cerebellar flocculus from the optic nerves in rabbits. In: Ito M, Tsukahara N, Kubota K, Yagi K (eds) Integrative control functions of the brain, vol 1. Kodansha Scientific, Tokyo/Elsevier, Amsterdam pp 93–95
Maekawa K, Takeda T, Kimura M (1981) Neural activity of nucleus reticularis tegmenti pontis-the origin of visual mossy fiber afferents to the cerebellar flocculus of rabbits. Brain Res 210: 17–30
Malmgren L, Olsson Y (1978) A sensitive method for histochemical demonstration of horseradish peroxidase in neurons following retrograde axonal transport. Brain Res 148: 279–294
Meesen H, Olszewski J (1949) A cytoarchitectonic atlas of the rhombencephalon of the rabbit. Karger, Basel
Mesulam M-M (1978) Tetramethylbenzidine for horseradish peroxidase neurochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neuronal afferents and efferents. J Histochem Cytochem 26: 106–117
Mesulam M-M, Rosene DL (1979) Sensitivity in horseradish peroxidase neurochemistry: a comparative and quantitative study of nine methods. J Histochem Cytochem 27: 763–773
Miyashita Y (1981) Differential roles of the climbing and mossy fiber visual pathways in vision-guided modification of the vestibuloocular reflex. In: Flohr H, Precht W (eds) Lesion induced neuronal plasticity in sensorimotor systems. Springer, Berlin Heidelberg New York, pp 305–313
Miyashita Y, Ito M, Jastreboff PJ, Maekawa K, Nagao S (1980) Effects upon eye movements of rabbits induced by severance of the mossy fiber visual pathway to the cerebellar flocculus. Brain Res 198: 210–215
Miyashita Y, Nagao S (1981) Signal content of Purkinje cell responses in rabbit's cerebellar flocculus to optokinetic stimuli. J Physiol Soc Jpn 43: 318
Pompeiano O, Mergner T, Corvaja N (1978) Commissural, perihypoglossal and reticular afferent projections to the vestibular nuclei in the cat. An experimental anatomical study with the method of the retrograde transport of horseradish peroxidase. Arch Ital Biol 116: 130–172
Precht W, Strata P (1980) On the pathway mediating optokinetic responses in vestibular nuclear neurons. Neuroscience 5: 777–787
Streit P, Reubi JC (1977) A new and sensitive staining method for axonal transported horseradish peroxidase in the pigeon visual system. Brain Res 126: 530–537
Yamamoto M (1979) Topographical representation in rabbit cerebellar flocculus for various inputs from the brain stem investigated by means of retrograde axonal transport of horseradish peroxidase. Neurosci Lett 12: 29–34
Yamamoto M, Shimoyama I (1977) Differential localization of rabbit's flocculus Purkinje cells projecting to the medial and superior vestibular nuclei, investigated by means of horseradish peroxidase retrograde axonal transport. Neurosci Lett 5: 279–283
Graybiel AM, Hartweig EA (1974) Some afferent connections of the oculomotor nucleus in the cat: an experimental study with tracer techniques. Brain Res 81: 543–551
Kotchabhakdi N, Hoddevik GH, Walberg F (1978) Cerebellar afferent projection from the perihypoglossal nuclei: an experimental study with the mothod of the retrograde transport of horseradish peroxidase. Exp Brain Res 31: 13–29
Steiger HJ, Büttner-Ennever JA (1979) Oculomotor nucleus afferents in the monkey demonstrated with horseradish peroxidase. Brain Res 60: 1–15
Yingcharoen K, Rinvik E (1982) Branched projections from the nucleus prepositus hypoglossi to the oculomotor nucleus and the cerebellum. A retrograde fluorescent double-labeling study in the cat. Brain Res 246: 133–136
Author information
Authors and Affiliations
Additional information
Supported by NIH National Research Service Award 1 F32 NS06331
Rights and permissions
About this article
Cite this article
Balaban, C.D. A projection from nucleus reticularis tegmenti pontis of Bechterew to the medial vestibular nucleus in rabbits. Exp Brain Res 51, 304–309 (1983). https://doi.org/10.1007/BF00237207
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00237207