Summary
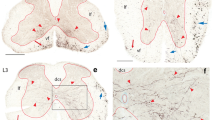
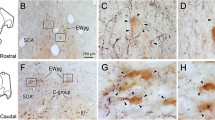
These experiments tested hypotheses about the logic of reticulospinal and reticuloreticular controls over deep back muscles by examining descending efferent and contralateral projections of the sites within the medullary reticular formation (MRF) that evoke EMG responses in lumbar axial muscles upon electrical stimulation. In the first series of experiments, retrograde tracers were deposited at gigantocellular reticular nucleus (Gi) sites that excited the back muscles and in the contralateral lumbar spinal cord. The medullary reticular formation contralateral to the Gi stimulation/deposition site was examined for the presence of single- and double-labeled cells from these injections. Tracer depositions into Gi produced labeled cells in the contralateral Gi and Parvocellular reticular nucleus (PCRt) whereas the lumbar injections retrogradely labeled cells only in the ventral MRF, indicating that separate populations of medullary reticular cells project to the opposite MRF and the lumbar cord. In the second series of experiments the precise relationships between the location of neurons retrogradely labeled from lumbar spinal cord depositions of the retrograde tracer, Fluoro-Gold (FG) and effective stimulation tracks through the MRF were examined. The results indicate that the Gi sites that are most effective for activation of the back muscles are dorsal to the location of retrogradely labeled lumbar reticulospinal cells. To verify that cell bodies and not fibers of passage were stimulated, crystals of the excitatory amino acid agonist, N-methyl-d-asparate (NMDA) were deposited at effective stimulation sites in the Gi. NMDA decreased the ability of electrical stimulation to activate back muscles at 5 min postdeposition, indicating a local interaction of NMDA with cell bodies at the stimulation site. In the third series of experiments, electrical thresholds for EMG activation along a track through the MRF were compared to cells retrogradely labeled from FG deposited into the cervical spinal cord. In some experiments, Fast Blue was also deposited into the contralateral lumbar cord. Neurons at low threshold points on the electrode track were labeled following cervical depositions, indicating a direct projection to the cervical spinal cord. The lumbar depositions, again, labeled cells in MRF areas that were ventral to the locations of effective stimulation sites, primarily on the opposite side of the medulla. In addition, the lumbar depositions back-filled cells in the same cervical segments to which the Gi neurons project. These results suggest that one efferent projection from effective stimulation sites for back muscle activation is onto propriospinal neurons in the cervical cord, which in turn project to lumbar cord levels. In a final series of experiments, a stimulating electrode track through the MRF again identified low threshold and ineffective sites for activating lumbar epaxial EMG. Fluoro-Gold was deposited in the contralateral MRF (MRFc) at a low threshold stimulation site for activating back muscles on that side. Retrogradely labeled cells surrounded effective, but not ineffective, stimulation sites along the electrode track in the MRF. Thus, another projection from effective stimulation sites is to effective stimulation sites in the opposite MRF. These results suggest that neurons in Gi whose stimulation most effectively activates back muscle EMG do not project directly to the lumbar cord, but relay to cervical cord neurons, which in turn project onto lumbar neurons. The MRF commissural connections presumably amplify this descending MRF control of axial back muscles.
Similar content being viewed by others
Abbreviations
- ECu:
-
external cuneate
- FB:
-
fast blue
- FG:
-
fluorogold
- Gi:
-
gigantocellular reticular nucleus
- GiA:
-
gigantocellular reticular nucleus, alpha
- GiV:
-
gigantocellular reticular nucleus, ventral
- icp:
-
inferior cerebellar peduncle
- IO:
-
inferior olive
- LL:
-
lateral longissimus
- ML:
-
medial longissimus
- mlf:
-
medial longitudinal fasciculus
- MRF:
-
medullary reticular formation
- MRFc:
-
medullary reticular formation, contralateral
- MVN:
-
medial vestibular nucleus
- PCRt:
-
parvocellular reticular formation
- PGi:
-
paragigantocellular nucleus
- PrH:
-
prepositus hypoglossal nucleus
- py:
-
pyramidal tract
- R:
-
rhodamine microspheres
- Sol:
-
nucleus of the solitary tract
- Sp5:
-
spinal trigeminal nucleus
- 7:
-
facial nucleus
References
Basbaum AI, Clanton CH, Fields HI (1978) Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol 178: 209–224
Brink EE, Pfaff DW (1981) Supraspinal and segmental input to lumbar epaxial motoneurons in the rat. Brain Res 226: 43–60
Brink EE, Morrell JI, Pfaff, DW (1979) Localization of lumbar epaxial motoneurons in the rat. Brain Res 170: 23–41
Brink EE, Modianos DT, Pfaff DW (1980) Ablations of lumbar epaxial musculature: effects on lordosis behavior of female rats. Brain Behav Evol 17: 67–88
Chaouch A, Menetrey D, Bender D, Besson JM (1983) Neurons at the origin of the medial component of the bulbopontine spinoreticular tract in the rat: an anatomical study using horseradish peroxidase retrograde transport. J Comp Neurol 214: 309–320
Cottingham SL, Femano PA, Pfaff DW (1987) Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of the axial muscle EMG. Exp Neurol 97: 704–724
Cottingham SL, Femano PA, Pfaff BW (1988) Vestibulospinal and reticulospinal interactions in the activation of back muscle EMG in the rat. Exp Brain Res 73: 198–208
Engberg I, Flatman JA, Lambert JDC (1979) The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol (Lond) 288: 227–261
Femano PA, Schwartz-Giblin S, Pfaff DW (1984a) Brain stem reticular influences on lumbar axial muscle activity. I. Effective sites. Am J Physiol 246: R389–395
Femano PA, Schwartz-Giblin S, Pfaff DW (1984b) Brain stem reticular influences on lumbar axial muscle activity. II. Temporal aspects. Am J Physiol 246: R396–401
Fox JE (1970) Reticulospinal neurones in the rat. Brain Res 23: 35–40
Garcia-Rill E, Skinner RD (1987) The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res 411: 1–12
Hayes NL, Rustioni A (1981) Descending projections from brainstem and sensorimotor cortex to spinal enlargements in the cat. Exp Brain Res 41: 89–107
Holstege G, Kuypers HGJM (1982) The anatomy of brain stem pathways to the spinal cord in ca. A labeled amino acid tracing study. Prog Brain Res 57: 145–175
Holstege JC, Kuypers HGJM (1987) Brainstem projections to lumbar motoneurons in rat-I. An ultrastructural study using autoradiography and the combination of autoradiography and horseradish peroxidase histochemistry. Neurosci 21: 345–367
Illert M, Jankowska E, Lundberg L, Odutola A (1981) Integration in descending motor pathways controlling the forelimb in the cat. 7. Effects from the reticular formation on C3-C4 propriospinal neurons. Exp Brain Res 42: 269–281
Ito K, McCarley RW (1987) Physiological studies of brainstem reticular connectivity. I. Responses of mPRF neurons to stimulation of bulbar reticular formation. Brain Res 409: 97–110
Jankowska E, Lundberg A (1981) Interneurones in the spinal cord. Trends Neurosci 4: 230–233
Jones BE, Yang T-Z (1985) The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242: 56–92
Keizer K, Kuypers HGJM (1984) Distribution of corticospinal neurons with collaterals to lower brain stem reticular formation in cat. Exp Brain Res 54: 107–120
Kevetter GA, Willis WD (1983) Collaterals of spinothalamic cells in the rat. J Comp Neurol 215: 453–464
Kow L-M, Montogomery MO, Pfaff DW (1977) Effects of spinal cord transections on lordosis reflex in female rats. Brain Res 123: 75–88
Lipski J, Bellingham MC, West MJ, Pilowsky P (1988) Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Meth 26: 169–179
Lloyd DPC (1941) Activity in neurons of the bulbospinal correlation system. J Neurophysiol 4: 115–134
Martin GF, Vertes RP, Waltzer R (1985) Spinal projections of the gigantocellular reticular formation in the rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp Brain Res 58: 154–162
Matsuyama K, Ohta Y, Mori S (1988) Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by the anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 460: 124–141
McCarley RW, Ito K, Rodrigo-Angulo ML (1987) Physiological studies of brainstem reticular connectivity. II. Responses of mPRF neurons to stimulation of mesencephalic and contralateral pontine reticular formation. Brain Res 409: 111–127
Menetrey D, DePommery J, Roudier F (1985) Propriospinal fibers reaching the lumbar enlargement in the rat. Neurosci Lett 58: 257–261
Mitani A, Ito K, Mitani Y, McCarley RW (1988) Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. J Comp Neurol 268: 546–566
Modianos D, Pfaff DW (1979) Medullary reticular formation lesions and lordosis reflex in female rats. Brain Res 171: 334–338
Monaghan DT, Olverman HJ, Nguyen L, Watkins JC, Cotman CW (1988) Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci 85: 9836–9840
Newman BD (1985) Distinguishing rat brainstem reticulospinal nuclei by their neuronal morphology. I. Medullary nuclei. J Hirnforsch 26: 187–226
Nudo RJ, Masterton RB (1988) Descending pathways to the spinal cord: a comparative study of 22 mammals. J Comp Neurol 277: 53–79
Ohta Y, Mori S, Kimura H (1988) Neuronal structures of the brainstem participating in postural suppression in cats. Neurosci Res 5: 181–202
Pechura CM, Liu RPC (1986) Spinal neurons which project to the periaqueductal gray and medullary reticular formation via axon collaterals: a double label fluorescence study in the rat. Brain Res 374: 357–361
Peterson BW, Maunz RA, Pitts NG, Mackel RG (1975) Patterns of projection and branching of reticulospinal neurons. Exp Brain Res 23: 333–351
Peterson BW, Pitts NG, Fukushima K (1979) Reticulospinal connections with limb and axial motoneurons. Exp Brain Res 36: 1–20
Pfaff DW, Schwartz-Giblin S (1988) Cellular mechanisms of female reproductive behaviors. In: The Physiology of Reproduction, E. Knobill, J Neill (eds). Raven Press, NY 1487–1568
Robbins A, Schwartz-Giblin S, Pfaff DW (1988) Reticulo-reticular and reticulo-spinal connections affecting EMG activity in rat back muscles. Soc Neurosci 14: 184
Robbins A, Schwartz-Giblin S, Pfaff DW (1990) Ascending and descending projections to medullary reticular formation sites which activate deep lumbar back muscles in the rat. Exp Brain Res 80: 463–474
Rye DB, Lee HG, Saper CB, Warner BH (1988) Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 269: 315–341
Schenkel E, Siegel JM (1989) REM sleep without atonia after lesions of the medial medulla. Neurosci Lett 98: 159–165
Schwartz-Giblin S, Femano PA, Pfaff DW (1984) Axial EMG and intervertebral length gauge responses during lordosis behavior in rats. Exp Neurol 85: 297–315
Shapovalov AI, Gurevitch N (1970) Monosynaptic and disynaptic reticulospinal actions on lumbar motoneurons of the rat. Brain Res 21: 249–263
Shen P, Arnold AP, Micevych PE (1990) Supraspinal projections to the ventromedial lumbar spinal cord in adult male rats. J Comp Neurol 300: 263–272
Sirkin DW, Feng AS (1987) Autoradiographic study of descending pathways from the pontine reticular formation and the mesencephalic trigeminal nucleus in the rat. J Comp Neurol 256: 483–493
Skinner RD, Remmel RS, Minor LB (1984) Monosynaptic activation of long descending propriospinal neurons from the lateral vestibular nucleus and the medial longitudinal fasciculus. Exp Neurol 86: 462–472
Skinner RD, Nelson R, Griebel M, Garcia-Rill E (1989) Ascending projections of long descending propriospinal tract (LPDT) neurons. Brain Res Bull 22: 253–258
Takakusaki K, Ohta Y, Mori S (1989) Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res 74: 11–23
Tebecis AK (1973) Transmitters and reticulospinal neurons. Exp Neurol 40: 297–308
Tebecis AK, Ishikawa I (1973) Glycine and GABA as inhibitory transmitters in the medullary reticular formation. Pflugers Arch 338: 273–278
Verburgh CA, Kuypers HGJM (1987) Branching neurons in the cervical spinal cord: a retrograde fluorescent double-labeling study in the rat. Exp Brain Res 68: 565–578
Vertes RP (1988) An anterograde and retrograde anatomical analysis of reticulo-reticular connections in the rat. Neurosci Abst 14: 1183
Zemlan FP, Pfaff DW (1979) Topographical organization in medullary reticulospinal systems as demonstrated by the horseradish peroxidase technique. Brain Res 174: 161–166
Zemlan FP, Kow L-M, Pfaff DW (1983) Effect of interruption of bulbospinal pathways on lordosis, posture, and locomotion. Exp Neurol 81: 177–194
Zemlan FP, Behbehani MM, Beckstead RM (1984) Ascending and descending projections from the nucleus magnocellularis and reticularis gigantocellularis: an autoradiographic and horseradish peroxidase study in the rat. Brain Res 292: 207–220
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robbins, A., Schwartz-Giblin, S. & Pfaff, D.W. Reticulospinal and reticuloreticular pathways for activating the lumbar back muscles in the rat. Exp Brain Res 92, 46–58 (1992). https://doi.org/10.1007/BF00230382
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230382