Summary
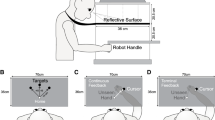
Hand trajectory, tangential velocity and acceleration, time and distance until peak velocity and reaction time were analyzed during the process of learning a skilled, visually-guided arm movement. Primates were trained to move a cursor with a manipulandum from a start box to target boxes displayed on a horizontal video screen during control conditions and when the relationship (gain) between the cursor and manipulandum was altered. The animals adapted to the altered feedback over 100–200 trials. A subsequent testing phase with randomly interspersed trials using the control gain demonstrated that the animals had modified their movements appropriately for the novel gain. Examination of the kinematics revealed that in adapting to a novel gain, primates scaled movement amplitude, tangential velocity, acceleration, and duration appropriately for the distance the hand needed to travel. Yet time to peak velocity was kept constant. Reaction time also remained unchanged for three of the four animals. Movements were performed in two phases, the first from movement onset to peak velocity and the second from peak velocity until the end of the movement. During the first phase the shape of the trajectory and velocity profile were stereotypic and without evidence of any corrections, consistent with this phase being essentially open loop. However, corrections occurred in the second phase and we propose visual feedback was used to correct for the difference in hand/cursor position. Learning appeared to involve utilizing the errors from previous trials to modify the early feedforward phase of subsequent trials. Peak tangential velocity, total movement duration and distance reached at peak tangential velocity all scaled linearly with the total movement distance required at each gain. Based on regression analyses, for none of these variables were the changes in learning completely adequate to compensate for total distance required. However, distance to peak velocity scaled with peak velocity in relation to the control gain. The results show that non-human primates adopt a consistent strategy when learning to scale a multi-joint movement. The metrics of the movement scaled yet the time to peak velocity remained constant, suggesting independent control of time and amplitude. Keeping time to peak velocity constant as well as the scaling of peak velocity with distance to peak velocity are viewed as ways to simplify the learning process.
Similar content being viewed by others
References
Abend W, Bizzi E, Morasso P (1982) Human arm trajectory formation. Brain 105: 331–348
Atkeson CG (1989) Learning arm kinematics and dynamics. Ann Rev Neurosci 12: 157–183
Beaubaton D, Grangetto A, Paillard J (1978) Contribution of positional and movement cues to visuomotor reaching in splitbrain monkey. In: Russell I, van Hoff MW, Berlucchi G (eds) Structure and function of cerebral commissures. University Park Press, Baltimore MD, 30: 371–384
Beggs WDA, Howarth CI (1972) The movement of the hand towards a target. Q J Exp Psychol 24: 448–453
Bernstein N (1967) The coordination and regulation of movements. Pergamon, Oxford
Bullock D, Grossberg S (1988) Neural dynamics of planned arm movements: emergent invariants and speed-accuracy properties during trajectory formation. Psychol Rev 95: 49–90
Enoka RM (1984) Muscular control of a learned movement: the speed control system hypothesis. Exp Brain Res 51: 135–145
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391
Fitts PM, Seeger CM (1953) S-R compatibility: spatial characteristics of stimulus and response codes. J Exp Psychol 46: 199–210
Flowers KA (1976) Visual ‘closed-loop’ and ‘open-loop’ characteristics of voluntary movement in patients with parkinsonism and intention tremor. Brain 99: 269–310
Freund HJ, Buedingen HJ (1978) The relationship between speed and amplitude of the fastest voluntary contraction of human arm muscles. Exp Brain Res 31: 1–12
Georgopoulos AP (1986) On reaching. Ann Rev Neurosci 9: 147–70
Georgopoulos AP, Kalaska JF, Massey JT (1981) Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophysiol 46: 725–743
Ghez C, Vicario D (1978) The control of rapid limb movement in the cat. II. Scaling of isometric force adjustments. Exp Brain Res 33: 191–202
Gilbert PFC, Thach WT (1977) Purkinje cell activity during motor learning. Brain Res 128: 309–328
Gonshor A, Melvill Jones G (1976) Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol 256: 381–414
Gordon J, Ghez C (1987) Trajectory control in targeted force impulses II. Pulse height control. Exp Brain Res 67: 241–252
Gottlieb G, Corcos D, Agarwal D (1989) Strategies for the control of voluntary movements with one mechanical degree of freedom. Behav Brain Sci 12: 189–250
Hogan N (1984) An organizing principle for a class of voluntary movements. JM Neurosci 4: 2745–2754
Hollerbach J, Flash T (1982) Dynamic interactions between limb segments during planar arm movement. Biol Cybern 44: 67–77
Keele SW, Posner MI (1968) Processing of visual feedback in rapid movements. J Exp Psychol 77: 155–158
Lacquaniti F, Soechting JF (1982) Coordination of arm and wrist motion during a reaching task. J Neurosci 2: 399–408
Lisberger SG (1988) The neural basis for learning of simple motor skills. Science 242: 728–735
Macpherson JM (1988) Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol 60: 204–217
Morasso P (1981) Spatial control of arm movements. Exp Brain Res 42: 223–227
Nagasaki H (1989) Asymmetric velocity and acceleration profiles of human arm movements. Exp Brain Res 74: 319–326
Nelson WL (1983) Physical principles for economies of skilled movements. Biol Cybern 46: 135–147
Ojakangas CL, Onstott DK, Tam DC, Ebner TJ (1988) Changes in hand kinematics during a quantifiable learning paradigm in primates. Soc Neurosci Abstr 14: 860
Optican LM, Miles FA (1985) Visually induced adaptive changes in primate saccadic oculomotor control signals. J Neurophys 54: 940–958
Sasaki K, Gemba H (1982) Development and change of cortical field potentials during learning processes of visually initiated hand movements in the monkey. Exp Brain Res 48: 429–437
Shapiro DC, Walter CB (1986) An examination of rapid positioning movements with spatiotemporal constraints. J Motor Behav 18: 373–395
Soechting JF (1984) Effect of target size on spatial and temporal characteristics of a pointing movement in man. Exp Brain Res 54: 121–132
Soechting JF, Lacquaniti F (1981) Invariant characteristics of a pointing movement in man. J Neurosci 1: 710–720
Soechting JF, Terzuolo CA (1987) Organization of arm movements: motion is segmented. Neuroscience 23: 39–51
Tam D (1987) Correlation of cerebellar Purkinje cell activity with the kinematics of a voluntary visually-guided closed-loop movement and interactions among Purkinje cells. Doctoral thesis. University of MN, Minneapolis
Thompson RF (1986) The neurobiology of learning and memory. Science 233: 941–947
Viviani P, Terzuolo C (1982) Trajectory determines movement dynamics. Neuroscience 7: 431–437
Wang J, Kim JH, Ebner TJ (1987) Climbing fiber afferent modulation during a visually-guided, multi-joint arm movement in the monkey. Brain Res 410: 323–329
Zelaznik HN, Hawkins B, Kisselburgh L (1983) Rapid visual feed back processing in single-aiming movements. J Motor Behav 15: 217–236
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ojakangas, C.L., Ebner, T.J. Scaling of the metrics of visually-guided arm movements during motor learning in primates. Exp Brain Res 85, 314–323 (1991). https://doi.org/10.1007/BF00229409
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229409