Summary
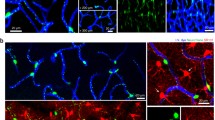
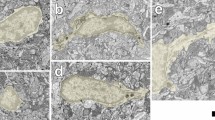
Nucleoside diphosphatase (IDPase), localized using inosine diphosphate as substrate, allows the selective staining of blood vessels and cells of vascular origin, such as macrophages and microglia, whereas the neuroglial, the neuronal and the pigment epithelial cells remain unstained. The staining pattern observed in the retina of mouse, rat, cat and monkey are similar; some apparent quantitative differences reflect species differences in the distribution of retinal microvasculature. At the electron-microscopic level, most of the enzyme activity in the blood vessels appears to be located along the outer wall. The cell membrane, parts of the smooth endoplasmic reticulum and the nuclear membrane in the microglial perikarya appear positive; profiles of microglial processes are intensely stained.
In the developing eyes of rats and mice, the blood vessels are stainable from the earliest stage of their appearance. An array of amoeboid cells precede the growing blood vessels and spread out over the future vascularized part of the retina. These cells eventually develop characteristic microglial features, and extend many elongated and branched processes between the neuroepithelial cells while remaining in contact with, or in close proximity to, the blood vessels. Intense IDPase activity in the microglial cells, in contrast to the absence of the enzyme in the neuroglial Müller cells, suggests that microglia are involved in phosphate metabolism and indicates functional compartmentalization within the glial tissue lying between the blood retinal barrier and the retinal neurons.
Similar content being viewed by others
References
Allen JM (1963) The properties of Golgi associated nucleoside diphosphatase and thiamine pyrophosphatase. I. Cytochemical analysis. J Histochem Cytochem 11:529–541
Bhattacharjee J (1976) Developmental changes of carbonic anhydrase in the retina of the mouse: a histochemical study. Histochem J 8:63–70
Bhattacharjee J, Sanyal S (1975) Developmental origin and early differentiation of retinal Müller cells in mice. J Anat 120:367–372
Bignami A, Dahl D (1979) The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to glial fibrillary acidic (GFA) protein. Exp Eye Res 28:63–69
Boycott BB, Hopkins JM (1981) Microglia in the retina of monkey and other mammals; its distinction from other types of glia and horizontal cells. Neuroscience 6:679–688
Büssow H (1980) The astrocytes in the retina and optic nerve head of mammals: A special glia for the ganglion cell axons. Cell Tissue Res 206:367–378
Choi BH (1981) Hematogenous cells in the central nervous system of developing human embryos and fetuses. J Comp Neurol 196:683–694
Cunha-Vas J (1980) Sites and functions of the blood-retinal barriers. In: Cunha-Vas JG (ed) The blood-retinal barriers, NATO Advanced Study Institutes series. Vol. 32, Plenum, New York, pp 101–117
Eisenfeld AJ, Bunt-Milam AH, Sarthy PV (1984) Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci 25:1321–1328
Engerman RL, Meyer RK (1965) Development of retinal vascula-ture in rats. Am J Ophthalmol 60:628–641
Fujita S, Kitamura T (1976) Origin of brain macrophages and the nature of the microglia. In: Zimmerman HM (ed) Progress in neuropathology, Grune and Stratton, New York, pp 1–50
Goldfischer S, Essner E, Schiller B (1971) Nucleoside diphosphatase and thiamine pyrophosphatase activities in the endoplasmic reticulum and Golgi apparatus. J Histochem Cytochem 19:349–360
Hogan MJ, Feeney L (1963) The ultrastructure of the retinal vessels. III. Vascular-glial relationships. J Ultrastruct Res 9:47–64
Hume DA, Perry VH, Gordon S (1983) Immunohistochemical localization of a macrophage specific antigen in developing mouse retina — phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol 97:253–257
Ibrahim MZH, Khreis Y, Koshayan DS (1974) The histochemical identification of microglia. J Neurol Sci 22:211–233
Imamoto K, Leblond CP (1977) Presence of labeled monocytes, macrophages, and microglia in association with a stab wound of the brain after an injection of bone marrow cells labeled with 3H-uridine into rats. J Comp Neurol 174:255–280
Imamoto K, Leblond CP (1978) Radioautographic study of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol 180:139–164
Imamoto K, Fujiwara R, Nagai T, Maeda T (1982) Distribution and fate of macrophagic ameboid cells in the rat brain. Arch Histol Jpn 45:505–518
Kitamura T, Miyake T, Fujita S (1984) Genesis of resting microglia in the gray matter of mouse hippocampus. J Comp Neurol 226:421–433
Konigsmark BW, Sidman RL (1963) Origin of brain macrophages in the mouse. J Neuropathol Exp Neurol 22:643–676
Lessell S, Kuwabara T (1964) Phosphatase histochemistry of the eye. Arch Ophthalmol 71:851–860
Ling EA (1981) Ultrastructure and peroxidase cytochemistry of macrophages present in the retina of postnatal rats. Arch Histol Jpn 44:167–176
Ling EA (1982) A light microscopic demonstration of amoeboid microglia and microglial cells in the retina of rats of various ages. Arch Histol Jpn 45:37–44
Linser P, Moscona AA (1979) Induction of glutamine synthetase in embryonic neural retina: localization in Muller fibres and dependence on cell interactions. Proc Natl Acad Sci USA 76:6476–6480
Linser PJ, Sorrentino M, Moscona AA (1984) Cellular compart-mentalization of carbonic anhydrase and glutamine synthetase in developing and mature mouse neural retina. Dev Brain Res 13:65–71
Murabe Y, Sano Y (1981) Thiamine pyrophosphatase activity in the plasma membrane of microglia. Histochemistry 71:45–52
Murabe Y, Sano Y (1982) Morphological studies on neuroglia. VI. Postnatal development of microglial cells. Cell Tissue Res 225:469–485
Murabe Y, Sano Y (1983) Morphological studies on neuroglia. VII. Distribution of “brain macrophages” in brains of neonatal and adult rats, as determined by means of immunohistochemistry. Cell Tissue Res 229:85–95
Novikoff AB, Goldfischer S (1961) Nucleoside diphosphatase activity in the Golgi apparatus and its usefulness for cytological studies. Proc Natl Acad Sci USA 47:802–810
Oehmichen M (1980) Enzyme histochemical differentiation of neuroglia and microglia: a contribution to the cytogenesis of microglia and globoid cells. Pathol Res Pract 168:344–373
Ogden TE (1978) Nerve fiber layer astrocytes of the primate retina: morphology, distribution, and density. Invest Ophthalmol Vis Sci 17:499–510
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Riepe RE, Norenberg MD (1977) Müller cell localization of glutamine synthetase in rat retina. Nature 268:654–655
Riepe RE, Norenberg MD (1978) Glutamine synthetase in developing rat retina — immunohistochemical study. Exp Eye Res 27:435–444
Rio-Hortega PD (1932) Microglia. In: Penfield W (ed) Cytology and cellular pathology of the nervous system, Vol. 2, Paul B Hoeber, New York, pp 481–534
Sanyal S (1972) Changes of lysosomal enzymes during hereditary degeneration and histogenesis of retina in mice. Histochemie 29:28–36
Sanyal S, De Ruiter A, Dees C (1984) Light dependent accumulation of macrophages at the photoreceptor-pigment epithelial interface in the retina of albino mice. Experientia 40:852–854
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Terubayashi H, Murabe Y, Fujisawa H, Itoi M, Ibata Y (1984) Enzymehistochemical identification of microglial cells in the rat retina: light and electron microscopic studies. Exp Eye Res 39:595–603
Wolff HH (1969) Über die Entwicklung des Enzymmusters der Rattenretina. Histochemie 17:11–29
Wolter JR (1957) Perivascular glia of the blood vessels of the human retina. Am J Ophthalmol 44:766–773
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sanyal, S., De Ruiter, A. Inosine diphosphatase as a histochemical marker of retinal microvasculature, with special reference to transformation of microglia. Cell Tissue Res. 241, 291–297 (1985). https://doi.org/10.1007/BF00217173
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217173