Abstract
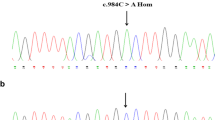
To seek the possible molecular defect in a patient with deficient factor X plasma procoagulant activity, factor X gene exons and splice junctions were subjected to heteroduplex analyses and sequencing. A mutation in exon 2 was confirmed as substitution of A by G at nucleotide position 206, coding for Gly instead of a Glu which is a normal precursor for γ-carboxylated glutamic acid (Gla) at amino acid position 14. An abolished TaqI restriction site was used to indicate homozygosity of the defect, but occurrence of a gene deletion with attendant heterozygosity could not be excluded. The deletion of a Gla residue could affect the Ca2+-binding properties of factor X or confer a flexibility interfering with the interactive properties of the light chain. The defect could explain the decreased functional activity of circulating factor X and the mild bleeding tendency of the propositus.
Similar content being viewed by others
References
Bell GI, Karam JH, Rutter WJ (1981) Polymorphic DNA region adjacent to the 5′ end of the human insulin gene. Proc Natl Acad Sci USA 78: 5759–5763
Chattopadhyay A, Fair DS (1989) Molecular recognition in the activation of human blood coagulation factor X. J Biol Chem 264: 11035–11043
Diuguid DL, Rabiet M-J, Furie BC, Furie B (1989) Molecular defects of factor IX Chicago-2 (Arg145→His) and prothrombin Madrid (Arg271→Cys): arginine mutations that preclude zymogen activation. Blood 74: 193–200
Fair DS, Plow EF, Edgington TS (1979) Combined functional and immunochemical analysis of normal and abnormal human factor X. J Clin Invest 64: 884–894
Fujikawa K, Titani K, Davie EW (1975) Activation of bovine factor X (Stuart factor): conversion of activation of factor Xaα to Xaβ. Proc Natl Acad Sci USA 72: 3359–3363
Fung MR (1988) Molecular genetics of blood coagulation factor X. Thesis, University of British Columbia, Canada
Jackson CM (1984) Factor X. In: Spaet TH (ed) Progress in hemostasis and thrombosis, vol 7. Grune and Stratton, Philadelphia, pp 55–109
James HL, Girolami A, Fair DS (1991) Molecular defect in coagulation factor XFriuli results from a substitution of serine for proline at position 343. Blood 77: 317–323
Leytus SP, Foster DC, Kurachi K, Davie EW (1986) Gene for factor X: a blood coagulation factor whose gene organization is essentially identical with that of factor IX and protein C. Biochemistry 25: 5098–5102
Liles DK, Lefkowitz J, Monroe DM, Roberts HR (1992) Corresponding γ-carboxyglutamyl (Gla) residues of factor IX (F IX) and factor X (F X) have different functional consequences. Blood 80: 163a
Mierendorf RC, Pfeffer D (1987) Direct sequencing of denatured plasmid DNA. Methods Enzymol 152: 556–562
Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T (1989) Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Proc Natl Acad Sci USA 86: 2766–2770
Pfeiffer RA, Ott R, Gilgenkrantz S, Alexandre P (1982) Deficiency of coagulation factors VII and X associated with deletions of a chromosome 13(q34): evidence from two cases with 46,XY,t(13;Y)(q11;q34). Hum Genet 62: 358–360
Ratcliffe JV, Furie B, Furie BC (1993) The importance of specific γ-carboxyglutamic acid residues in prothrombin: evaluation by site-specific mutagenesis. J Biol Chem 268: 24339–24345
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491
Suttie JW (1985) Vitamin K-dependent carboxylase. Ann Rev Biochem 54: 459–477
Wallmark A, Parson P, Ljung R, Monroe D, High K (1991) Molecular defect (Gla26→Asp) and its functional consequences in a hereditary factor X deficiency (factor X “Malmo 4”). Blood 78: 60a
Watzke HH, Lechner K, Roberts HR, Reddy SV, Welch DJ, Friedman P, Mahr G, Jagadeeswaran P, Monroe DM, High K (1990) Molecular defect (Gla+l4→Lys) and its functional consequences in a hereditary factor X deficiency (factor X “Vorarlsberg”). J Biol Chem 265: 11982–11989
Zhang L, Jhingan A, Castellino FJ (1992) Role of individual γ-carboxyglutamic acid residues of activated human protein C in defining its in vitro anticoagulant activity. Blood 80: 942–952
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, D.J., Thompson, A.R. & James, H.L. Factor XKetchikan: a variant molecule in which Gly replaces a Gla residue at position 14 in the light chain. Hum Genet 95, 212–214 (1995). https://doi.org/10.1007/BF00209404
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00209404