Abstract
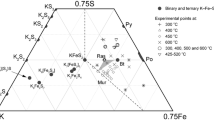
Pentlandite has a wide compositional range in the Fe-Ni-Co-S system and the Fe-Ni-S system. The metal : sulfur atomic ratio is approximately 9 : 8. The Co content of pentlandite from the Kamaishi ore deposit and one from the Outokumpu ore deposit (Knop et al. 1965) varies from a Co-free to nearly a Co9S8 composition. Pentlandite forms a complete solid solution between (Fe, Ni)9±xS8 and Co9±xS8 in the 600°–300°C temperature range. On the other hand, it is presumed that the pentlandite solid solution decomposes into two fields toward the (Fe, Ni)9S8 and Co9S8 members at 200°C. The field of solid solution at 500°C is the most extensive and includes all of the other solid-solution fields. The metal : sulfur ratio of the solid solution varies systematically, i.e., the Fe-rich field sinks toward the Fe-Ni-Co plane away from the M9S8 section, and contrarily the Ni-rich field shifts toward the sulfur apex. The d044 value decreases with increasing S, Ni, or Co contents in pentlandite. The Kamaishi pentlandites, Iwate Prefecture, Japan, lie within or close to the solid-solution field at 300°C, showing a very good correlation with the Co : Ni ratio of the homogeneous natural pyrrhotite phase.
Similar content being viewed by others
References
Barton, P.B., Jr., Skinner, B.J.: Sulfide stabilities. In: Geochemistry of hydrothermal ore deposits., 2nd. Edition. Barns, H.L. (ed). John Wiley & Sons, New York, pp. 278–403 (1967)
Bell, P.M., England, J.L., Kullerud, G.: Pentlandite pressure effect on breakdown. Carnegie Inst. Washington Yearb. 63:206–207 (1964)
Clark, T., Naldrett, A. J.: The distribution of Fe and Ni between synthetic olivine and sulfide at 900°C. Econ. Geol. 67: 939–952 (1972)
Craig, J.R.: Pentlandite composition. Carnegie Inst. Washington Yearb. 65:329 (1966)
Craig. J.R.: Pyrite-pentlandite and other low temperature assemblages in the Fe-Ni-S system. Amer. Jour. Sci. 273A:496–510 (1973)
Edwards, W.E., Hudson, D.R.: An interpretive study of a nickeliron sulfide ore intersection, Lunnon Shoot, Kambalda, Western Australia. Econ. Geol. 67:1075–1092 (1972)
Fleet, M.E.: The crystal structure of NiS. Acta Crystallogr. A24:390–397 (1972)
Harris, D.C., Nickel, E.H.: Pentlandite composition and association in some mineral deposits. Can. Mineral. 11:861–878 (1972)
Hawley, J.E.: The Sudbury ores: their mineralogy and origin. Can. Mineral. 7:1–207 (1962)
Huhma, A., Huhma, M.: Contribution of the geology and geochemistry of the Outokumpu region. Bull. Geol. Soc. Finland. 42:57–88 (1970)
Imai, H., Fijiki, Y.: Study on the nickel- and cobalt-bearing sulfide minerals from the Komori mine by means of Electron Probe Microanalyzer. Mining Geol. 13:333–338 (1963)
Imai, N., Mariko, T., Kaneda, H., Shiga, Y.: Compositional variation of pentlandites in copper sulfide ores from the Kamaishi mine, Iwate Prefecture, Japan. Mining Geol: 263–276 (1980)
Kaneda, H.: Genesis of the Kamaishi deposits, Iwate Prefecture, with special reference to the phase relations in the Cu-Fe-Ni-Co-S system. Unpub. Dr. Thesis., The Univ. of Tokyo, Tokyo (1981)
Kaneda, H., Shoji, T., Takenouchi, S.: Sulfide mineralization of the Shinyama ore deposit, Kamaishi mine, Iwate Prefecture. Mining Geol. 30:169–180 (1980)
Kilburn, L.C., Wilson, H.D.B., Graham, A.R., Ogura, Y., Coats, C.J.A., Scoates, R.F.: Nickel sulfide ores related to ultrabasic intrusion in Canada. Econ. Geol. Mono. 4:276–293 (1969)
Knop, O., Ibrahim, M. A.: Chalcogenides of the transition elements II. Existence of the π phase in the M9S8 section of the system Fe-Co-Ni-S. Can. Jour. Chem. 39:297–317 (1961)
Knop, O., Ibrahim, M.A., Sutarno: Chalcogenides and the transition element IV. Pentlandite natural phase. Can. Mineral. 8:291–316 (1965)
Kojonen, K.: Experiments on synthetic pentlandite. N. Jh. Miner. Abh. 126:133–135 (1976)
Koubo, O., Huhma, M., Vuorelainein, Y.: A natural cobalt analogue of pentlandite. Amer Mineral. 44:897–900 (1959)
Kullerud, G.: The Fe-Ni-S system. Carnegie Inst. Washington Yearb. 62:175–189 (1963)
Lindqvist, M., Lundqvist, D., Westergren, A.: The crystal structure of Co9S8 and of pentlandite (Ni, Fe)9S8. Svensk Kem. Tidskr. 48:156–160 (1936)
Misra, K.C., Fleet, M.E.: The chemical compositions of synthetic and natural pentlandite assemblages. Econ. Geol. 68:518–539 (1973)
Newhouse, W.H.: Equilibrium relations of pyrrhotite and pentlandite. Econ. Geol. 22:288–299 (1929)
Petruk, W., Harris, D.C., Stewart, J.M.: Langisite, a new mineral and the rare minerals cobalt pentlandite, siegenite, parkerite and bronoite from the Langis mine, Cobalt-Gowganda, Ontario. Can. Mineral. 9:597–616 (1969)
Rajamani, V., Prewitt, C.T.: Crystal chemistry of natural pentlandite. Can. Mineral. 12:178–187 (1973)
Ramdohr, P.: The ore minerals and their intergrowths. Pergamon Press. Oxford, pp. 495–601 (1969)
Shewman, R.W., Clark, L.A.: Pentlandite phase relations in the Fe-Ni-S system and notes on the monosulfide solid solution. Can. Jour. Earth Sci. 7:67–85 (1970)
Shoji, T., Sasaki, N.: Geochemisty of cobalt and nickel in the Shimokawa copper deposit, Hokkaido. Jour. Min. Metal. Inst. Japan. 96:523–528 (1981)
Toulmin, P., III, Barton, P.G., Jr.: A thermodynamic study of pyrite and pyrrhotite. Geochim. Cosmochim. Acta. 28:641–671 (1964)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaneda, H., Takenouchi, S. & Shoji, T. Stability of pentlandite in the Fe-Ni-Co-S system. Mineral. Deposita 21, 169–180 (1986). https://doi.org/10.1007/BF00199797
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00199797