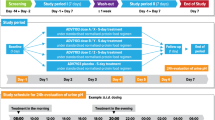
Abstract
Mucosal oral therapeutic system (MOTS) is a controlled-release osmotic system for oral cavity therapy. MOTS (nystatin) is designed to deliver approximately 200,000 units of nystatin over several hours. A crossover study was conducted in five healthy volunteers to evaluate the amount of nystatin released (based on residual drug content) when the system is held in the mouth for 30 min, 1 h, and 2 h, and to compare these concentrations with those achieved with a Mycostatin (nystatin) pastille.
An average of 37% of the nystatin content was released intra-orally from MOTS during 2 h in the mouth, which was very similar to the percentage delivered in vitro. Mean salivary drug concentrations were as follows: 279 μg·ml−1 at 30 min; 654 μg·ml−1 after 1 h; and 532 μg·ml−1 at 2 h. These concentrations consistently exceeded those produced by the pastille at the same time points. Fifteen minutes after placement of the pastille in the mouth (i.e., immediately after its dissolution) mean nystatin concentrations reached 746 μg·ml−1 but fell rapidly to 13.2 μg·ml−1 at 30 min, 7.2 μg·ml−1 at 1 h, and 5.6 μg·ml−1 at 2 h.
The study demonstrates that MOTS maintains high salivary nystatin concentrations throughout a 2 h dosing interval.
Similar content being viewed by others
References
Aldridge H, Aldridge J, Walker DM (1983) The intraoral distribution and duration in saliva of amphotericin B. J Dent Res 62: 428
American Hospital Formulary Service (1993) Drug Information 93. American Society of Hospital Pharmacists, Bethesda, Md, pp 2190–2192
Bouckaert S, Schautteet H, Lefebvre RA, Remon JP, Clooster R van (1992) Comparison of salivary miconazole concentrations after administration of a bioadhesive slow-release buccal tablet and an oral gel. Eur J Clin Pharmacol 43: 137–140
Martin MV, Farrelly PJ, Hardy P (1986) An investigation of the efficacy of nystatin for the treatment of chronic atrophic candidasis (denture sore mouth). Br Dent J 160: 201–204
Pedersen M, Rassing MR (1990) Miconazole chewing gum as a drug delivery system. Application of solid dispersion technique and lecithin. Drug Dev Ind Pharm 16: 2015–2030
Pedersen M, Rassing MR (1991) Miconazole chewing gum as a drug delivery system. Test of release promoting additives. Drug Dev Ind Pharm 17: 411–420
Physicians' Desk Reference, 47th edn (1993) Medical Economics Co, Montvale, NJ, pp 751
Sande MA, Kapusnik-Uner JE, Mandell GL (1990) Chemotherapy of microbial diseases. Antimicrobial agents: general considerations. In: Gilman AG, Rall TW, Nies AS, Taylor P (eds) The pharmacological basis of therapeutics, 8th edn. Pergamon Press, New York, pp 1032
Theeuwes F, Yum SI, Haak R, Wong P (1991) Systems for triggered, pulsed, and programmed delivery. In: Temporal control of drug delivery. Ann NY Acad Sci, pp 428–440
The United States Pharmacopeia XXII (1989) Biological tests and assays. Antibiotics — microbial assays. The United States Pharmacopeial Convention, Rockville, Md, sect 81, pp 1488–1493
Vries-Hospers HG de, Waaij D van der (1980) Salivary concentrations of amphotericin B following its use as an oral lozenge. Infection 8: 63–65
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Encarnacion, M., Chin, I. Salivary nystatin concentrations after administration of an osmotic controlled release tablet and a pastille. Eur J Clin Pharmacol 46, 533–535 (1994). https://doi.org/10.1007/BF00196111
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196111