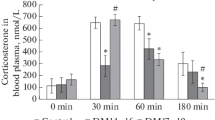
Abstract
In order to determine the incidence of maternal glucocorticoids on morphological parameters in fetal development, we performed optic and electron microscopic analysis of the cerebral cortex of fetuses of 16 and 20 days of gestation, from control (C) and pregnant rats bilaterally adrenalectomized on day 1 of gestation (ADX). We also studied fetuses 20 days old from pregnant rats betamethasone-injected on days 15, 16 and 17 (BET), and adrenalectomized on day 1 and betamethasone-injected on days 15, 16 and 17 (ADX+BET). Absence of maternal glucocorticoids during gestation caused, in fetuses 16 and 20 days old, a marked increase of cellular density, laxity of tissue and lower cellular maturation in comparison with the control group. Beta-methasone injected into sham-operated animals (BET) caused a slight advance in relation to controls in developmental parameters such as cellular density, maturation and synapse formation. Betamethasone injection into adrenalectomized animals prevented the lower degree of maturation characteristic of the adrenalectomized group, although an increase of cellular density could be detected. The cerebral cortex from fetuses of 16 days of gestation from adrenalectomized mothers also showed an increase of cellular density as compared with the control group. These results show that glucocorticoids participate in prenatal rat brain in control mechanisms of cellular division and maturation.
Similar content being viewed by others
Abbreviations
- ADX :
-
adrenalectomized animals
- ADX+BET :
-
adrenalectomized, betamethasone-injected animals
- BET :
-
betamethasone-injected animals
- C :
-
control animals
- CORT :
-
corticosterone
- CP :
-
cortical plate
- GC :
-
glucocorticoids
- GR :
-
glucocorticoid receptor
- I :
-
cortical layer I
- IZ :
-
intermediate zone
- LV :
-
lateral ventricle
- NE :
-
neuroepithelium
- Sp :
-
subplate
- SV :
-
subventricular layer
References
Alexis MN, Kitraki E, Spanou K, Stylianopoulou F, Sekeris CE (1989) Ontogeny of the glucocorticoid receptor in the rat brain. Adv Exp Med Biol 265:269–276
Altman J, Bayer S (1990) Horizontal compartmentation in the germinal matrices and intermediate zone of the embryonic rat cerebral cortex. Exp Neurol 107:36–47
Angevine JB, Sidman RL (1961) Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192:766–768
Arahuetes RM, Carretero V, Diebold Y, Rúa C (1991) Effects of bilateral adrenalectomy and prenatal betamethasone administration on fetal encephalic development. Biol Neonate 59:303–313
Barres BA, Raff MC (1994) Control of oligodendrocyte number in the developing rat optic nerve. Neuron 12:935–942
Barres BA, Lazar MA, Raff MC (1994) A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120:1097–1108
Bayer SA, Altman J (1991a) Neocortical development. Raven Press, New York, pp 11–29
Bayer SA, Altman J (1991b) Neocortical development. Raven Press, New York, pp 30–48
Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR (1993) Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 341:339–341
Beyer PE, Chernoff N (1986) The induction of supernumerary ribs in rodents: role of the maternal stress. Teratogenesis Carcinog Mutagen 6:419–429
Bohn MC (1980) Granule cell genesis in the hippocampus of rats treated neonatally with hydrocortisone. Neuroscience 5:2003–2012
Bohn MC, Lauder A (1978) Effects of neonatal hydrocortisone on rat cerebellar development. A morphological and autoradiographic study. Dev Neurosci 1:250–264
Carretero V, Arahuetes RM, Rúa C (1989) Influence of glucocorticoids in fetal encephalic development. J Endocrinol Invest 12[Suppl 2]:74
Catalano S, Killackey HP (1990) Ingrowth of thalamocortical axons into embryonic rat neocortex. Soc Neurosci Abstr 16:1007
Cintra A, Solfrini V, Bunnemann B, Okret S, Bortolotti F, Gustafsson J-A, Fuxe K (1993) Prenatal development of glucocorticoid receptor gene expression and immunoreactivity in the rat brain and pituitary gland: a combined in situ hybridization and immunocytochemical analysis. Neuroendocrinology 57:1133–1147
Cotterrell M, Balazs R, Johnson AL (1972) Effects of corticosteroids on the biochemical maturation of rat brain: postnatal cell formation. J Neurochem 19:2151–2167
Devenport LD (1979) Adrenal modulation of brain size in adult rats. Behav Neural Biol 27:218–221
Devenport LD, Devenport JA (1982) Effects of adrenal hormones on brain and body size. Physiol Psychol 10:399–404
Devenport LD, Devenport JA (1985) Adrenocortical hormones and brain growth: reversibility and differential sensitivity during development. Exp Neurol 90:44–52
Dupouy MJP, Coffigny H, Magre S (1975) Maternal and fetal corticosterone levels during late pregnancy in rats. J Endocrinol 65:347–352
Edwards CR, Benediktsson R, Lindsay RS, Seckl JR (1993) Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet 341:355–357
Gannon M, McEwen BS (1990) Calmodulin involvement in stress-and corticosterone-induced down-regulation of cyclic AMP-generating system in brain. J Neurochem 55:276–284
Golstein P, Ojcius DM, Young JD-E (1991) Cell death mechanisms and the immune system. Immunol Rev 121:29–45
Gould E, Woolley CS (1990) Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience 37:367–375
Gould E, Woolley CS (1991a) Adrenal steroids regulate postnatal development of the rat dentate gyrus. I. Effects of glucocorticoids on cell death. J Comp Neurol 313:479–485
Gould E, Woolley CS (1991b) Adrenal steroids regulate postnatal development of the rat dentate gyrus. II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol 313:486–493
Hashimoto H, Marystone JF, Greenough WT, Bohn MC (1989) Neonatal adrenalectomy alters dendriting branching of hippocampal granule cells. Exp Neurol 104:62–67
LaBorde JB, Hansen DK, Young JF, Sheehan DM, Holson RR (1992) Prenatal dexamethasone exposure in rats: effects of dose, age at exposure, and drug-induced hypophagia on malformations and fetal organ weights. Fundam Appl Toxicol 19:545–554
Lamour Y, Dutar P, Jobert A (1982) Topographic organization of basal forebrain neurons projecting to the rat cerebral cortex. Neurosci Lett 34:117–122
Landfield P, Baskin R, Pitler T (1981) Brain aging correlates: retardation by hormonal-pharmacological treatments. Science 214:581
Leret ML, González MI, Arahuetes RM (1993) Effect of maternal adrenal deprivation on the content of catecholamines in fetal brain. Life Sci 52:1609–1615
McEwen BS (1992) Steroid hormones: effect on brain development and function. Horm Res 37[Suppl 3]:1–10
McEwen BS, DeKloet ER, Rostene W (1986) Adrenal steroid receptors and actions in the nervous system. Physiol Rev 66:1121–1188
McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley C (1992a) Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry 31:177–199
McEwen BS, Gould EA, Sakai RR (1992b) The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry 160[Suppl 15]:18–24
McNeill TH, Masters JN (1991) Effect of chronic adrenalectomy on neuron loss and distribution of sulfated glycoprotein-2 in the dentate gyrus of adult rats. Exp Neurol 111:140–144
Meaney M, Sapolsky R, Aitken D, McEwen BS (1985) Tritiateddexamethasone binding in the limbic brain of the fetal rat. Dev Brain Res 23:297
Meyer JS (1981) Early adrenalectomy stimulates subsequent growth and development of the rat forebrain. Neurosci Abstr 7:289
Meyer JS (1983) Early adrenalectomy stimulates subsequent growth and development of rat brain. Exp Neurol 82:432–446
Meyer JS (1987) Prevention of adrenalectomy induced brain growth stimulation by corticosterone treatment. Physiol Behav 41:391–395
Meyer JS, Fairman KR (1985) Early adrenalectomy increases myelin content of the rat brain. Dev Brain Res 17:1–9
Miller MW (1986) The migration and neurochemical differentiation of gamma-aminobutyric acid (GABA)-immunoreactive neurons in rat visual cortex as demonstrated by a combined immunocytochemical-autoradiographic technique. Dev Brain Res 28:41–46
Munck A, Guyre PM, Holbrook NJ (1984) Physiological function of glucocorticoids in stress and their relation to pharmacological action. Endocr Rev 5:25–44
Paxinos G, Törk I, Tecott LH, Valentino KL (1991) Atlas of the developing rat brain. Academic Press, San Diego
Raedler E, Raedler A (1978) Autoradiographic study of early neurogenesis in rat neocortex. Anat Embryol 154:267–284
Reul JMH, DeKloet ER (1985) Two receptor systems for corticosterone in the rat brain: microdistribution and differential occupation. Endocrinology 117:2505–2511
Sapolsky RM (1990) Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res 86:13–22
Sapolsky RM, Meaney MJ (1986) Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev 11:65–76
Sapolsky RM, Stein-Behrens BA (1991) Long-term adrenalectomy causes loss of dentate gyrus and pyramidal neurons in the adult hippocampus. Exp Neurol 114:246–249
Sapolsky RM, Krey L, McEwen BS (1984) Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci USA 81:6174–6177
Sapolsky RM, Krey L, McEwen BS (1986) The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev 7:284–301
Selye H (1973) The evolution of the stress concept. Am Sci 61:692–699
Slotkin TA, Lappi SE, McCook EC, Tayyeb MI, Eylers JP, Seidler FJ (1992) Glucocorticoids and the development of neuronal function: effects of prenatal dexamethasone exposure on central noradrenergic activity. Biol Neonate 61:326–336
Sloviter RS, Valiquette G (1989) Selective loss of hipocampal granule cells in the mature rat brain after adrenalectomy. Science 243:535–538
Thomas TL, Devenport LD (1988) Site specificity of adrenalectomy-induced brain growth. Exp Neurol 102:340–345
Trejo JL, Arahuetes RM, Machín C, Rúa C (1992) Effects of early maternal adrenal deprivation on hippocampus and brain and cerebellar cortex during gestation. Electron Microscopy vol 3. EUREM 92:717
Ward IL, Weisz J (1984) Differential effects of maternal stress on circulating levels of corticosterone, progesterone and testosterone in the female rat fetuses and their mothers. Endocrinology 114:1635–1644
Yehuda R, Meyer JS (1991) Regional patterns of brain growth during the first three weeks following early ADX. Physiol Behav 49:233–237
Yehuda R, Fairman KR, Meyer JS (1989) Enhanced brain cell proliferation following early adrenalectomy in rats. J Neurochem 53:241–248
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trejo, J.L., Machín, C., Arahuetes, R.M. et al. Influence of maternal adrenalectomy and glucocorticoid administration on the development of rat cerebral cortex. Anat Embryol 192, 89–99 (1995). https://doi.org/10.1007/BF00186994
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00186994