Summary
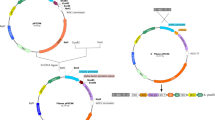
The human blood coagulation protein Factor XIIIa (FXIIIa) was expressed in Saccharomyces cerevisiae employing Escherichia coli-yeast shuttle vectors based on a 2-μ plasmid. Several factors affecting high production yield of recombinant FXIIIa were analysed. The use of the regulatable GAL-CYC1 hybrid promoter resulted in higher FXIIIa expression when compared with the constitutive ADCI promoter. Screening for suitable yeast strains for expression of FXIIIa under the transcriptional control of the GAL-CYC1 hybrid promoter revealed a broad spectrum of productivity. No obvious correlation between the expression rate and the genetic markers of the strains could be identified. The medium composition markedly influenced the FXIIIa expression rates. The expression of FXIIIa was strictly regulated by the carbon source. Glucose as the only sugar and energy source repressed the synthesis of FXIIIa, whereas addition of galactose induced FXIIIa expression. Special feeding schemes resulted in a productivity of up to 100 mg FXIIIa/l in shake flasks.
Similar content being viewed by others
References
Abe A, Wada T, Handa H, Nogi Y, Fukasawa T (1988) Efficient usage of a galactose-inducible expression vector for the production of heterologous protein in yeast. Agric Biol Chem 52:2035–2041.
Amann E, Abel K-J, Grundmann U, Okazaki H, Küpper HA (1988a) Synthesis of human FXIIIa in bacterial cells. Behring Inst Mitt 82:35–42.
Amann E, Ochs B, Abel K-J (1988b) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315.
Ammerer G (1983) Expression of genes in yeast using the ADCI promoter. Methods Enzymol 101:192–201.
Bitter GA, Egan KM (1984) Expression of heterologous genes in Saccharomyces cerevisiae from vectors utilizing the glyceraldehyde-3-phosphate dehydrogenase gene promoter. Gene 32:263–274.
Board PG (1979) Genetic polymorphisms of the a subunit of human coagulation factor XIII. Am J Hum Genet 31:116–124.
Bröker M (1987) Transformation of intact Schizosaccharomyces pombe cells with plasmid DNA. BioTechniques 5:516–518.
Bröker M, Bäuml O (1989) New expression vectors for the fission yeast Schizosaccharomyces pombe. FEBS Lett 248:105–110.
Bröker M, Ragg H, Karges HE (1987) Expression of human anti-thrombin III in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Biochim Biophys Acta 980:203–213.
Burnette WN (1981) “Western blotting”: electrophoretic transfer of proteins from sodium dodecylsulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203.
Cesareni G, Murray JAH (1987) Plasmid vectors carrying the replication origin of filamentous single-stranded phages. In: Setlow JK (ed) Genetic engineering, vol 9. Plenum Press, New York, pp 135–153.
Chung SI, Lewis MS, Folk JE (1974) Relationships of the catalytic properties of human plasma and platelet transglutaminases (activated blood coagulation factor XIII) to their subunit structures. J Biol Chem 249:940–950.
Das S, Kellermann E, Hollenberg CP (1984) Transformation of Kluyveromyces fragilis. J Bacteriol 158:1165–1167.
Grundmann U, Amann E, Zettlmeissl G, Küpper HA (1986) Characterization of cDNA coding for human factor FXIIIa. Proc Natl Acad Sci USA 83:8024–8028.
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmid DNA. J Mol Biol 166:557–580.
Holmes DS, Quigley M (1981) A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114:193–197.
Hopkins DJ, Betenbaugh MJ, Dhurjati P (1987) Effects of dissolved oxygen shock on the plasmid stability of recombinant Escherichia coli containing plasmid pKN401. Biotechnol Bioeng 29:85–91.
Ichinose A, Hendrickson LE, Fujikawa K, Davie EW (1986) Amino acid sequence of the subunit of human factor XIII. Biochemistry 25:6900–6906.
Johnston M (1987) A model fungal gene regulatory mechanism: the Gal genes of Saccharomyces cerevisiae. Microbiol Rev 51:458–476.
Karges HE (1984) Blood coagulation factor XIII: determination by clot stability assays. In: Bergmeyer J, Grass M (eds) Methods of enzymatic analysis. Verlag Chemie, Weinheim, pp 400–405.
Karges HE, Clemens R (1988) Factor XIII: enzymatic and clinical aspects. Behring Inst Mitt 82:43–58.
Kingsman SM, Kingsman AJ, Mellor J (1987) The production of mammalian proteins in Saccharomyces cerevisiae. TibTech 5:54–57.
Kleiman MJ, Gingold EB, Stanbury PF (1986) The stability of yeast plasmid pJDB248 depends on growth rate of the culture. Biotechnol Lett 8:225–230.
Kniskern PJ, Hagopian A, Montgomery DL, Burke P, Dunn NR, Hofman KJ, Miller WJ, Ellis RW (1986) Unusually high-level expression of a foreign gene (hepatitis B virus core antigen) in Saccharomyces cerevisiae. Gene 46:135–141.
Laughton A, Gesteland RF (1984) Primary structure of the Saccharomyces cerevisiae GAL4 gene. Mol Cell Biol 4:260–267.
Lee GM, Song KB, Rhee SK, Han MH (1986) Plasmid stability and growth of recombinant Saccharomyces cerevisiae producing hepatitis B virus surface antigen. Biotechnol Lett 8:385–390.
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N. Y.
Mead DJ, Gardner DCJ, Oliver SG (1986) Enhanced plasmid stability of a 2 μ-based recombinant plasmid in diploid yeast. Biotechnol Lett 8:391–396.
Parker C, DiBiasio (1987) Effect of growth rate and expression level on plasmid stability in Saccharomyces cerevisiae. Biotechnol Bioeng 29:215–221.
Purvis IJ, Andrew AJE, Loughlin L, Brown AJP (1987) The effect of alterations within the 3′-untranslated region of the pyruvate kinase messenger RNA upon its stability and translation in Saccharomyces cerevisiae. Nucleic Acids Res 15:7951–7962.
Rinas U, Risse B, Jaenicke R, Abel K-J, Zettlmeissl G (1990) Denaturation-renaturation of fibrin-stabilizing factor XIII a-chain isolated from human placenta. Properties of the native and reconstituted protein. Biol Chem Hoppe-Seyler 371:49–56.
Rothstein RJ (1983) One-step gene disruption in yeast. Methods Enzymol 101:202–211.
Santiago TC, Purvis IJ, Bettany AJE, Brown AJP (1986) The relationship between mRNA stability and length in Saccharomyces cerevisiae. Nucleic Acids Res 14:8347–8360.
Schultz LD, Tanner J, Hofmann KJ, Emini EA, Condra HJ, Jones RE, Kieff E, Ellis RE (1987) Expression and secretion in yeast of a 400-kDa envelope glycoprotein derived from Epstein-Barr virus. Gene 54:113–123.
Sherman F, Fink GR, Hicks JB (1983) Methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N. Y.
Stepien PP, Brousseau R, Wu R, Narang S, Thomas DY (1983) Synthesis of a human insulin gene. VI. Expression of the synthetic proinsulin in yeast. Gene 24:289–297.
Suzuki K, Matsiu K, Ito S, Fujita K, Matsumoto H (1988) Polymorphism of the a subunit of a coagulation factor XIII: evidence for subtypes of the FXIIIa*I and FXIIIa*2 alleles. Am J Hum Genet 43:170–174.
Takahashi N, Takahashi Y, Putnam FW (1986) Primary structure of the blood coagulation factor XIIIa (fibrinolase, transglutaminase) from human placenta. Proc Natl Acad Sci USA 83:8019–8023.
Wieslander L (1979) A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem 98:305–309.
Author information
Authors and Affiliations
Additional information
Offprint requests to: M. Bröker
Rights and permissions
About this article
Cite this article
Bröker, M., Bäuml, O., Göttig, A. et al. Expression of the human blood coagulation protein Factor XIIIa in Saccharomyces cerevisiae: dependence of the expression levels from host-vector systems and medium conditions. Appl Microbiol Biotechnol 34, 756–764 (1991). https://doi.org/10.1007/BF00169346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169346