Abstract
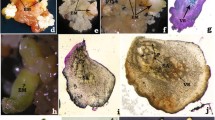
A successful system of somatic embryogenesis is described for the forest tree Ocotea catharinensis Mez., which used mature zygotic embryo explants cultured on a modified Murashige and Skoog (MS) medium with activated charcoal, at 25°C in the dark. A medium composed of MS supplemented with 2% (w/v) sucrose, 0.3% (w/v) activated charcoal (AC), 362 μM 2,4-dichlorophenoxyacetic acid (2,4-d) and 0.8% (w/v) Technical Agar Grade III was used for multiplication of embryogenic cultures. Development up to the globular-stage was achieved using Lloyd and McCown woody plant medium (WPM) with 2.0% sucrose, 0.3% AC, 181 μM 2,4-d and 0.8% Technical Agar Grade III. Significant effects of media pH on differentiation of early pro-embryogenic Ocotea cell aggregates were found. Low pH of media (ca. 3–4) appeared to prevent differentiation of proembryogenic cell aggregates whereas higher pH levels (ca. 5–5.5) favoured the formation of globular structures. Once globular structures formed, they developed further to form cotyledonary somatic embryos, under the same set of culture conditions. Successful conversion of these somatic embryos to plantlets was achieved after culture on a medium composed of 1/2-strength WPM (minerals only) with 2% sucrose, 0.3% AC, 0.8% Technical Agar Grade III and 90.5 μM 2,4-d, followed by transfer to a medium composed of 1/2-strength WPM (minerals only) with 2% sucrose, 0.8% Technical Agar Grade III and 0.905 μM 2,4-d and 1.4 μM gibberellic acid, in a 16-h photoperiod regime.
Similar content being viewed by others
References
Ebert A & Taylor HF (1990) Assessment of the changes of 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tiss. Org. Cult. 20: 165–172
Gamborg OL, Miller RA & Ojima K (1968) Plant cell cultures. I. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158
George EF & Sherrington PD (1984) Plant Propagation by Tissue Culture (709 p). Exegetics Ltd, Basingstoke
Hartmann HT, Kester DE & Davies FT (1990) Plant Propagation: Principles and Practices, (647 p.). Prentice-Hall International Ltd., London
Hu C & Wang P (1986) Embryo culture: technique and application. In: Evans DA, Sharp WR & Ammirato PV (Eds) Handbook of Plant Cell Culture, Vol 4 (pp 43–96). Macmillan Publishers, London
Kirby EG, Leustek T & Lee MS (1987) Nitrogen nutrition. In: Bonga JM & Durzan DJ (Eds) Cell and Tissue Culture in Forestry, Vol 1 (pp 67–88). Kluwer Academic Publishers, Lancaster
Kochba J, Spiegel-Roy P & Safran H (1972) Adventive plants from ovule and nucelli in Citrus. Planta 106: 237–245
Lloyd G & McCown B (1981) Commercially-feasible micro-propagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Prop. Soc. (1980) 30: 421–427
Minocha SC (1987) pH of the medium and the growth and metabolism of cells in culture. In: Bonga JM & Durzan DJ (Eds) Cell and Tissue Culture in Forestry, Vol 1 (pp 125–141). Kluwer Academic Publishers, Lancaster
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Randi AM (1982) Estudo preliminar sobre inibidores de germinação em frutos de Miconia cinammomifolia e Ocotea puberula. In: Anais do Congresso Nacional Sobre Espécies Nativas, Campos do Jordão, Setembro 1982. Silvicultura em S. Paula 16(1): 238–242
Redenbaugh K, Fujii JA & Slade D (1988) Encapsulated plant embryos. In: Mizrahi A (Ed) Biotechnology in Agriculture (pp 255–258). Alan R. Liss Inc., New York
Rose D & Martin SM (1975) Effect of ammonium on growth of plant cells (Ipomoea sp) in suspension cultures. Can. J. Bot. 53: 1942–1949
Sargent PA & King J (1974) Investigations of growth-promoting factors in conditioned soybean root cells and in the liquid medium in which they grew: ammonium, glutamine and aminoacids. Can. J. Bot. 52: 1747–1755
Smith DL & Krikorian AD (1990a) Low external pH replaces 3,4-d in maintaining and multiplying 2,4-D-initiated embryogenic cells of carrot. Physiol Plant 80: 329–336
Smith DL & Krikorian AD (1990b) pH control of carrot somatic embryogenesis. In: Nijkamp HJJ, Van der Plas LHW & Van Aartrijk J (Eds) Progress in Plant Cellular and Molecular Biology (pp 449–453). Kluwer Academic Publishers, Lancaster
Smith DL & Krikorian AD (1990c) Somatic embryo production from excised, wounded zygotic carrot embryos on hormone-free medium: evaluation of the effects of pH, ethylene and activated charcoal. Plant Cell Rep. 9: 34–37
Sturion JA & Iede ET (1982) Influência da profundidade de semeadura, cobertura do canteiro e sombreamento na formação de mudas de Ocotea porosa (Nees) Liberato Barroso (Imbuia). Documentos, Unidade Regional de Pesquisa Florestal Centro-Sul, Embrapa 10: 71–79
Tulecke W (1987) Somatic embryogenesis in woody perennials. In: Bonga JM & Durzan DJ (Eds) Cell and Tissue Culture in Forestry, Vol 1 (pp 61–91). Kluwer Academic Publishers, Lancaster
Veliky IA & Rose D (1973) Nitrate and ammonium as nitrogen nutrients for plant cell cultures. Can. J. Bot. 51: 1837–1844
Viana AM, Moura-Costa PH & Mantell SH (1990) High frequency somatic embryogenesis of Ocotea catharinensis. In: Abstracts VIIth International Congress on Plant Tissue and Cell Culture, Amsterdam, 1990 (261 p.)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moura-Costa, P.H., Viana, A.M. & Mantell, S.H. In vitro plantlet regeneration of Ocotea catharinensis, an endangered Brazilian hardwood forest tree. Plant Cell Tiss Organ Cult 35, 279–286 (1993). https://doi.org/10.1007/BF00037282
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037282