Abstract
Alzheimer’s disease (AD) is a prevalent and debilitating neurological condition with no curable therapies in the pipeline. Alzheimer’s disease-related neurodegeneration negatively influences various brain cells, including astrocytes, neurons, and microglia. Interactions between healthy neural cell types and AD hallmarks: Aβ plaques and tau fibril deposits have been shown to produce detrimental cellular stresses and deterioration in brain functions. Astrocytes help to regulate nutritional balance, cerebral blood flow, tissue healing, and the blood–brain barrier. They engage in the CNS’s inflammatory/immune responses. Evidence also suggests that astrocytes have neuroprotective and neurotoxic effects based on the disease stage and microenvironment. Changes in astrocyte function have been observed in individuals with early-onset Alzheimer’s disease, displaying disproportions in gliotransmission, neurotransmitter homeostasis, astroglial atrophy, disruptions in synaptic associations, neuroinflammation, and neurodegeneration. Moreover, the presence of Aβ plaques appears to impact astrocytes, affecting calcium levels and pro-inflammatory activity via RAGE-NF-kB pathway. The conventional treatment approaches for AD-related neuropathology are not anticipated to be comprehensive. The rationale for this may be that AD treatment involves a therapeutic strategy to minimize its cascading recurrence, and most drugs are inefficient at developing effective responses. Hence, researchers are keen on exploring the roles of astrocytes and their molecular pathways to identify potent therapies in AD. This review provides a broad approach, emphasizing the roles of astrocytes in healthy brains and their pathological changes in AD, as well as potential astrocyte-related therapy regimens. Novel strategies comprising astrocytes and aimed at alleviating oxidative stress and neuroinflammation in AD have also been presented.
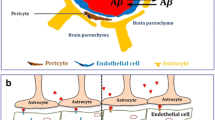
Graphical Abstract

The graphical abstract demonstrates the neuropathological implications of Alzheimer’s disease on several neural sub-populations, i.e. neurons, astrocytes, microglia, and oligodendrocytes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 2 ERK:
-
Extracellular signal-regulated kinase
- ABCC1:
-
ATP-binding cassette subfamily C member 1
- AD:
-
Alzheimer’s disease
- AICD:
-
Amyloid precursor protein intracellular domain
- ALS:
-
Amyotrophic lateral sclerosis
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- APP:
-
Amyloid precursor protein
- ATP:
-
Adenosine triphosphate
- Aβ:
-
Amyloid-beta
- B CN:
-
Calcineurin
- BBB:
-
Blood–brain barrier
- BMP:
-
Bone morphogenetic proteins
- CBF:
-
Cerebral blood flow
- CFB:
-
Complement factor
- CNS:
-
Central nervous system
- CNTF:
-
Ciliary neurotrophic factor
- Cx43:
-
Gap junction alpha-1 protein
- DAM:
-
Disease-associated macrophages
- DG:
-
Dentate gyrate
- EAAT2:
-
Excitatory amino acid transporter
- EGFR:
-
Epidermal growth factor receptor
- GABA:
-
Gamma-aminobutyric acid
- GFAP:
-
Glial fibrillary acidic protein
- GH:
-
Growth hormone
- GLAST1:
-
Glutamate transporter
- GLT-1:
-
l-glutamate transporter
- GSH:
-
Glutathione
- GSK3β:
-
Glycogen synthase kinase-3 beta
- HMGB1:
-
High mobility group box 1 protein
- IkB-α:
-
Nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitor
- ILGF-1:
-
Insulin-like growth factor 1
- JAK:
-
Janus kinase
- LRPs:
-
Lipoprotein receptor-related protein
- MAO-B:
-
Monoamine oxidase B
- MAP-2:
-
Microtubule-associated protein 2
- MEK1/MAP2K:
-
Mitogen-activated protein kinase
- MMP:
-
Mitochondrial membrane potential
- MX1S:
-
MX dynamin-like GTPase 1
- MyD88:
-
Myeloid differentiation primary response 88
- NADPH:
-
Reduced nicotinamide adenine dinucleotide phosphate
- NFAT:
-
Nuclear factor of activated T cells
- NF-kB:
-
Nuclear factor kappa B
- NFTs:
-
Interneuronal neurofibrillary tangles
- NICD:
-
Notch intracellular domain
- NMDAR:
-
N-methyl-D-aspartate receptor
- NOTCH:
-
Notch homolog 1, translocation-associated
- NOX:
-
NADPH-oxidized
- NPCs:
-
Neural precursor cells
- NRF2:
-
Nuclear factor-erythroid factor 2-related factor 2
- NSCs:
-
Neural stem cells
- OXPHOS:
-
Oxidative phosphorylation
- PAR:
-
Poly(ADP-ribose) polymers
- PARP:
-
Poly (ADP-ribose) polymerase-1
- PD:
-
Parkinson’s disease
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator
- PHF:
-
Paired helical fragments
- ptau:
-
Phosphorylated tau
- RAGE:
-
Receptor for advanced glycation end products
- RBP-J:
-
Recombination signal binding protein for immunoglobulin kappa J region
- ROCK inhibitor:
-
Rho-kinase inhibitor
- ROS:
-
Reactive oxygen species
- R-SMADs:
-
Receptor-regulated Smads
- S100B:
-
S100 calcium-binding protein B
- sAPP-β:
-
Soluble amyloid precursor protein beta
- SGZ:
-
Sub-granular zones
- sNMDAR:
-
Synaptic N-methyl-D-aspartate receptor
- SOCS3:
-
Suppressor of cytokine signalling 3
- SR:
-
Scavenger receptor
- STAT:
-
Signal transducer and activator of transcription
- TFAM:
-
Mitochondrial transcription factor A
- TG:
-
Transgenic mice
- TGIF:
-
Transforming growth-interacting factor
- TLR4:
-
Toll-like receptor 4
- TNF-α:
-
Tumour necrosis factor alpha
- TRF:
-
Thyrotropin-releasing factor
- TTP:
-
Alpha-tocopherol transfer protein
- U.S. FDA:
-
United States Food and Drug Administration
- V-SVZ:
-
Ventricular–sub-ventricular zones
- α-TCP:
-
Alpha-tocopherol
References
Abeti R, Abramov AY, Duchen MR (2011) Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain 134(Pt 6):1658–1672
Abramov AY, Canevari L, Duchen MR (2004) Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci 24(2):565–575
Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR (2005) Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci 25(40):9176–9184
Acaz-Fonseca E, Ortiz-Rodriguez A, Azcoitia I et al (2019) Notch signaling in astrocytes mediates their morphological response to an inflammatory challenge. Cell Death Dis 5:85
Alberdi E, Wyssenbach A, Alberdi M, Sánchez-Gómez MV, Cavaliere F, Rodríguez JJ, Verkhratsky A, Matute C (2013) Ca(2+)-dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12(2):292–302
Alves VS, Alves-Silva HS, Orts DJB, Ribeiro-Silva L, Arcisio-Miranda M, Oliveira FA (2019) Calcium signaling in neurons and glial cells: role of cav1 channels. Neuroscience 421:95–111
Angulo MC, Le Meur K, Kozlov AS, Charpak S, Audinat E (2008) GABA, a forgotten gliotransmitter. Prog Neurobiol 86(3):297–303
Arai H, Lee VM, Messinger ML, Greenberg BD, Lowery DE, Trojanowski JQ (1991) Expression patterns of beta-amyloid precursor protein (beta-APP) in neural and nonneural human tissues from Alzheimer’s disease and control subjects. Ann Neurol 30(5):686–693
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22(5):208–215
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81(4):728–739
Arendt T, Stieler JT, Holzer M (2016) Tau and tauopathies. Brain Res Bull 126(Pt 3):238–292
Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1(1):103–116
Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG (1994) Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 14(9):5559–5569
Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391(6664):281–285
Böck MC, Höfner G, Wanner KT (2020) N-Substituted nipecotic acids as (S)-SNAP-5114 analogues with modified lipophilic domains. ChemMedChem 15(9):756–771
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23(4):609–620
Bürkle A (2005) Poly(ADP-ribose). The most elaborate metabolite of NAD+. FEBS J 272(18):4576–4589
Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA (2009) Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci 29(47):14741–14751
Cao F, Hata R, Zhu P, Ma YJ, Tanaka J, Hanakawa Y, Hashimoto K, Niinobe M, Yoshikawa K, Sakanaka M (2006) Overexpression of SOCS3 inhibits astrogliogenesis and promotes maintenance of neural stem cells. J Neurochem 98(2):459–470
Carbone M, Duty S, Rattray M (2012) Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem Int 60(1):31–38
Chay KO, Nam Koong KY, Hwang S, Kim JK, Bae CS (2017) NADPH oxidase mediates β-amyloid peptide-induced neuronal death in mouse cortical cultures. Chonnam Med J 53(3):196–202
Chen S, Luo M, Zhao Y, Zhang Y, He M, Cai W, Liu A (2015) Fasudil stimulates neurite outgrowth and promotes differentiation in C17.2 neural stem cells by modulating notch signalling but not autophagy. Cell Physiol Biochem 36(2):531–541
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65(1):1–105
Darnell JE Jr, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264(5164):1415–1421
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27(5):457–464
Delekate A, Füchtemeier M, Schumacher T et al (2014) Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat Commun 5:5422
Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol 143(3):777–794
Emsley JG, Macklis JD (2006) Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol 2(3):175–186
Fatoba O, Itokazu T, Yamashita T (2021) Complement cascade functions during brain development and neurodegeneration. FEBS J 289(8):2085–2109
Floyd RA, Carney JM (1992) Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol 32(Suppl):22–27
Foster JB, Lashley R, Zhao F, Wang X, Kung N, Askwith CC, Lin L, Shultis MW, Hodgetts KJ, Lin CG (2019) Enhancement of tripartite synapses as a potential therapeutic strategy for Alzheimer’s disease: a preclinical study in rTg4510 mice. Alzheimers Res Ther 11(1):75
Fratiglioni L, De Ronchi D, Agüero-Torres H (1999) Worldwide prevalence and incidence of dementia. Drugs Aging 15(5):365–375
Fridovich I (1999) Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci 893:13–18
Furman JL, Norris CM (2014) Calcineurin and glial signaling: neuroinflammation and beyond. J Neuroinflammation 11:158
Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L et al (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349(6311):704–706
Gonzalez-Reyes RE, Rubiano MG (2018) Astrocyte’s RAGE: more than just a question of mood. Cent Nerv Syst Agents Med Chem 18(1):39–48
Guo MF, Zhang HY, Li YH, Gu QF, Wei WY, Wang YY, Zhang XJ, Liu XQ, Song LJ, Chai Z, Yu JZ, Ma CG (2020) Fasudil inhibits the activation of microglia and astrocytes of transgenic Alzheimer’s disease mice via the downregulation of TLR4/Myd88/NF-κB pathway. J Neuroimmunol 346:577284
Gupta S, Mishra K, Surolia A, Banerjee K (2011) Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition. PLoS One 6(11):e26674
Hefendehl J, LeDue J, Ko R et al (2016) Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat Commun 7:13441
Heneka MT, Carson MJ, El Khoury J, Landreth GE et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405
Henstridge CM, Tzioras M, Paolicelli RC (2019) Glial contribution to excitatory and inhibitory synapse loss in neurodegeneration. Front Cell Neurosci 13:63
Herceg Z, Wang ZQ (2001) Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res 477(1-2):97–110
Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM (1999) RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97(7):889–901
Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D et al (1995) The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270(43):25752–25761
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148(6):1204–1222
Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, Hess HF, Lippincott-Schwartz J, Liu Z (2019) Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177(6):1522–1535
Itoh S, Itoh F, Goumans MJ, Ten Dijke P (2000) Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem 267(24):6954–6967
Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Grünblatt E (2007) Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimers Dis 11(1):97–116
Jäkel S, Dimou L (2017) Glial cells and their function in the adult brain: a journey through the history of their ablation. Front Cell Neurosci 11:24
Jessen KR (2004) Glial cells. Int J Biochem Cell Biol 36(10):1861–1867. https://doi.org/10.1016/j.biocel.2004.02.023
Jo S, Yarishkin O, Hwang YJ, Chun YE et al (2014) GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med 20(8):886–896
Kaniyappan S, Chandupatla RR, Mandelkow EM, Mandelkow E (2017) Extracellular low-n oligomers of tau cause selective synaptotoxicity without affecting cell viability. Alzheimers Dement 13(11):1270–1291
Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10(9):698–712
Khan MI, Hasan F, Hasan Al Mahmud KA, Adnan A (2021) Domain focused and residue focused phosphorylation effect on tau protein: a molecular dynamics simulation study. J Mech Behav Biomed Mater 113:104149
Kidd M (1963) Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 197:192–193
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184
Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ (2009) Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323(5918):1211–1215
Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, Rutschman R, Murray PJ (2003) SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol 4(6):546–550
Larson EB, Kukull WA, Katzman RL (1992) Cognitive impairment: dementia and Alzheimer’s disease. Annu Rev Public Health 13:431–449
Lau CL, O’Shea RD, Broberg BV, Bischof L, Beart PM (2011) The Rho kinase inhibitor Fasudil up-regulates astrocytic glutamate transport subsequent to actin remodelling in murine cultured astrocytes. Br J Pharmacol 163(3):533–545
Laurence A, Pesu M, Silvennoinen O, O'Shea J (2012) JAK kinases in health and disease: an update. Open Rheumatol J 6:232–244
Lehre KP, Danbolt NC (1998) The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 18(21):8751–8757
Li T, Chen X, Zhang C, Zhang Y, Yao W (2019) An update on reactive astrocytes in chronic pain. J Neuroinflammation 16(1):140
Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu HC, Zheng H (2015) NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85(1):101–115
Liddelow SA (2019) Modern approaches to investigating non-neuronal aspects of Alzheimer’s disease. FASEB J 33(2):1528–1535
Lim DA, Alvarez-Buylla A (2016) The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol 8(5):a018820
Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E, Genazzani AA (2013) Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 61(7):1134–1145
Love S, Barber R, Wilcock GK (1999) Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain 122(Pt 2):247–253
Luna-Muñoz J, Chávez-Macías L, García-Sierra F, Mena R (2007) Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J Alzheimers Dis 12(4):365–375
Martínez-Zamudio RI, Ha HC (2014) PARP1 enhances inflammatory cytokine expression by alteration of promoter chromatin structure in microglia. Brain Behav 4(4):552–565
Martire S, Mosca L, d'Erme M (2015) PARP-1 involvement in neurodegeneration: a focus on Alzheimer’s and Parkinson’s diseases. Mech Ageing Dev 146-148:53–64
Masliah E, Terry RD, DeTeresa RM, Hansen LA (1989) Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett 103(2):234–239
Matyash V, Kettenmann H (2010) Heterogeneity in astrocyte morphology and physiology. Brain Res Rev 63(1-2):2–10
Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D (2003) NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci 6(10):1072–1078
Meraz-Ríos MA, Lira-De León KI, Campos-Peña V, De Anda-Hernández MA, Mena-López R (2010) Tau oligomers and aggregation in Alzheimer’s disease. J Neurochem 112(6):1353–1367
Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ (2000) Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101(5):499–510
Murgas P, Godoy B, von Bernhardi R (2012) Aβ potentiates inflammatory activation of glial cells induced by scavenger receptor ligands and inflammatory mediators in culture. Neurotox Res 22(1):69–78
Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging 25:663–674
Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T (1999) Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284(5413):479–482
Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge GL, Peineau S (2013) The role of JAK-STAT signaling within the CNS. JAKSTAT 2(1):e22925
Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45
Okamoto M, Gray JD, Larson CS, Kazim SF, Soya H, McEwen BS, Pereira AC (2019) Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl Psychiatry 8(1):153
Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp 71(2):281–299
Park JH, Ju YH, Choi JW, Song HJ et al (2019) Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer’s disease. Sci Adv 5(3):eaav0316
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 232(8):421–431
Perrig WJ, Perrig P, Stähelin HB (1997) The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc 45(6):718–724
Piccinin GL, Finali G, Piccirilli M (1990) Neuropsychological effects of L-deprenyl in Alzheimer’s type dementia. Clin Neuropharmacol 13(2):147–163
Pleiss MM, Sompol P, Kraner SD, Abdul HM, Furman JL, Guttmann RP, Wilcock DM, Nelson PT, Norris CM (2016) Calcineurin proteolysis in astrocytes: Implications for impaired synaptic function. Biochim Biophys Acta 1862(9):1521–1532
Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (2006) Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 26(27):7212–7221
Qin B, Cartier L, Dubois-Dauphin M, Li B, Serrander L, Krause KH (2006) A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging 27(11):1577–1587
Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB (2008) Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res 39:1–16
Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16(3):675–686
Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB (2005) Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433(7021):73–77
Sarlus H, Heneka MT (2017) Microglia in Alzheimer’s disease. J Clin Invest 127(9):3240–3249
Selvaraju TR, Khaza'ai H, Vidyadaran S, Abd Mutalib MS, Vasudevan R (2014) The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn J Basic Med Sci 14(4):195–204
Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X (2012) Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 120(3):419–429
Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, Finkel SI, Gwyther LP, Khachaturian ZS, Lebowitz BD, McRae TD, Morris JC, Oakley F, Schneider LS, Streim JE, Sunderland T, Teri LA, Tune LE (1997) Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 278(16):1363–1371
Sriram K, Benkovic SA, Hebert MA, Miller DB, O'Callaghan JP (2004) Induction of gp130- related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem 279(19):19936–19947
Szabó C, Ohshima H (1997) DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1(5):373–385
Takahashi K, Kong Q, Lin Y, Stouffer N, Schulte DA, Lai L, Liu Q, Chang LC, Dominguez S, Xing X, Cuny GD, Hodgetts KJ, Glicksman MA, Lin CL (2015) Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer’s disease. J Exp Med 212(3):319–332
Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T (2001) DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell 1(6):749–758
Tang BL (2009) Neuronal protein trafficking associated with Alzheimer disease: from APP and BACE1 to glutamate receptors. Cell Adhes Migr 3:118–128
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30(4):572–580
Trushina NI, Bakota L, Mulkidjanian AY, Brandt R (2019) The evolution of tau phosphorylation and interactions. Front Aging Neurosci 11:256
Turner PR, O’Connor K, Tate WP, Abraham WC (2003) Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 70(1):1–32
Tyzack GE, Hall CE, Sibley CR, Cymes T et al (2017) A neuroprotective astrocyte state is induced by neuronal signal EphB1 but fails in ALS models. Nat Commun 8(1):1164
Ulatowski L, Ghelfi M, West R, Atkinson J, Finno CJ, Manor D (2022) The tocopherol transfer protein TTP mediates Vitamin E trafficking between cerebellar astrocytes and neurons. J Biol Chem 298(3):101712
Vahedi G, Takahashi H, Nakayamada S, Sun HW, Sartorelli V, Kanno Y, O’Shea JJ (2012) STATs shape the active enhancer landscape of T cell populations. Cell 151(5):981–993
Varela G, Varona L, Anderson K, Sansoni J (2011) Alzheimer’s care at home: a focus on caregivers strain. Prof Inferm 64(2):113–117
Verkhratsky A (2019) Astroglial calcium signaling in aging and Alzheimer’s disease. Cold Spring Harb Perspect Biol 11(7):a035188
Vuong B, Hogan-Cann AD, Alano CC, Stevenson M, Chan WY, Anderson CM, Swanson RA, Kauppinen TM (2015) NF-κB transcriptional activation by TNFα requires phospholipase C, extracellular signal-regulated kinase 2 and poly(ADP-ribose) polymerase-1. J Neuroinflammation 12:229
Webster B, Hansen L, Adame A, Crews L, Torrance M, Thal L et al (2006) Astroglial activation of extracellular-regulated kinase in early stages of Alzheimer disease. J Neuropathol Exp Neurol 65:142–151
Wilkie CM, Barron JC, Brymer KJ, Barnes JR, Nafar F, Parsons MP (2021) The effect of GLT-1 upregulation on extracellular glutamate dynamics. Front Cell Neurosci 15:661412
Wu Z, Guo Z, Gearing M, Chen G (2014) Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s (corrected) disease model. Nat Commun 5:4159
Xiao Q, Du Y, Wu W, Yip HK (2010) Bone morphogenetic proteins mediate cellular response and, together with Noggin, regulate astrocyte differentiation after spinal cord injury. Exp Neurol 221(2):353–366
Xiu J, Nordberg A, Zhang JT, Guan ZZ (2005) Expression of nicotinic receptors on primary cultures of rat astrocytes and up-regulation of the alpha7, alpha4 and beta2 subunits in response to nanomolar concentrations of the beta-amyloid peptide(1-42). Neurochem Int 47(4):281–290
Yao GL, Kato H, Khalil M, Kiryu S, Kiyama H (1997) Selective upregulation of cytokine receptor subchain and their intracellular signalling molecules after peripheral nerve injury. Eur J Neurosci 9:1047–1054
Ye B, Shen H, Zhang J, Zhu YG, Ransom BR, Chen XC, Ye ZC (2015) Dual pathways mediate β-amyloid stimulated glutathione release from astrocytes. Glia 63(12):2208–2219
Yoshizumi M, Eisenach JC, Hayashida K (2012) Riluzole and gabapentinoids activate glutamate transporters to facilitate glutamate-induced glutamate release from cultured astrocytes. Eur J Pharmacol 677(1-3):87–92
Acknowledgment
All the schematic diagrams were made using Biorender.com software.
Conflicts of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Approval: Not applicable.
Consent to Participate: Yes.
Consent for Publication: Yes.
Availability of Data and Materials: Not applicable.
Competing Interests: None.
Funding: None.
Authors’ Contributions: All authors contributed to the study conception and design.
Acknowledgements: The authors acknowledge support from the Principal of Poona College of Pharmacy, Pune.
Disclosure of Potential Conflicts of Interest: Not applicable.
Research Involving Human Participants and/or Animals: Not applicable.
Informed Consent: Not applicable.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shareena, G., Kumar, D., Wu, D. (2023). Traversing Through the Trajectory of Pathogenic Astrocytes in Alzheimer’s Disease. In: Kumar, D., Patil, V.M., Wu, D., Thorat, N. (eds) Deciphering Drug Targets for Alzheimer’s Disease. Springer, Singapore. https://doi.org/10.1007/978-981-99-2657-2_8
Download citation
DOI: https://doi.org/10.1007/978-981-99-2657-2_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2656-5
Online ISBN: 978-981-99-2657-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)