Abstract
Malign tumor is one of the leading causes of human death worldwide, and its incidence increased rapidly year by year. Thus, it is of great importance to improve the strategy of early diagnosis for malign tumors. Aptamers are single-stranded oligonucleotide ligands with excellent chemical properties such as high affinity, easy synthesis, controllable chemical modifications, low immunogenicity, high stability, and fast tissue penetration. Therefore, aptamers are regarded as chemical antibodies and exhibit untapped potential for clinical applications. In this chapter, we highlight the advantages and properties of aptamer-based methods for cancer diagnosis, including cancer biomarker discovery, biosensing, and tumor imaging. We focus on describing several analyses approaches for protein biomarkers, circulating tumor cells, and cancer-related exosomes. The recent imaging techniques for tumor visual measurement are briefly expounded. Furthermore, the challenges and perspectives for the clinical application of aptamer-based methods are discussed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin 68(6):394–424
Hassan EM, Willmore WG, DeRosa MC (2016) Aptamers: promising tools for the detection of circulating tumor cells. Nucleic Acid Ther 26(6):335–347
Hanahan D, Weinberg Robert A (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Zichi D, Eaton B, Singer B, Gold L (2008) Proteomics and diagnostics: llet’s Get Specific, again. Curr Opin Chem Biol 12(1):78–85
Shigdar S, Qiao L, Zhou S-F, Xiang D, Wang T, Li Y, Lim LY, Kong L, Li L, Duan W (2013) RNA aptamers targeting cancer stem cell marker CD133. Cancer Lett 330(1):84–95
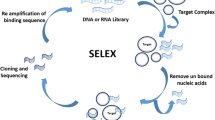
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Stoltenburg R, Reinemann C, Strehlitz B (2007) SELEX-A (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng 24(4):381–403
Keefe AD, Pai S, Ellington A (2010) Aptamers as therapeutics. Nat Rev Drug Discov 9(7):537–550
Mayer G (2009) The chemical biology of aptamers. Angew Chem Int Ed 48(15):2672–2689
Song S, Wang L, Li J, Fan C, Zhao J (2008) Aptamer-based biosensors. TrAC Trends Anal Chem 27(2):108–117
Cox JC, Ellington AD (2001) Automated selection of anti-protein aptamers. Biorg Med Chem 9(10):2525–2531
Liu J, Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109(5):1948–1998
Dalton WS, Friend SH (2006) Cancer biomarkers-an invitation to the table. Science 312(5777):1165–1168
Aebersold R, Anderson L, Caprioli R, Druker B, Hartwell L, Smith R (2005) Perspective: a program to improve protein biomarker discovery for cancer. J Proteome Res 4(4):1104–1109
Mallick P, Kuster B (2010) Proteomics: a pragmatic perspective. Nat Biotechnol 28(7):695
Jain KK, Jain KK (2010) The handbook of biomarkers. Springer
Hood LE, Omenn GS, Moritz RL, Aebersold R, Yamamoto KR, Amos M, Hunter-Cevera J, Locascio L, Participants W (2012) New and improved proteomics technologies for understanding complex biological systems: addressing a grand challenge in the life sciences. Proteomics 12(18):2773–2783
Ray P, Rialon-Guevara KL, Veras E, Sullenger BA, White RR (2012) Comparing human pancreatic cell secretomes by in vitro aptamer selection identifies cyclophilin B as a candidate pancreatic cancer biomarker. J Clin Invest 122(5):1734–1741
Pang X, Cui C, Wan S, Jiang Y, Zhang L, Xia L, Li L, Li X, Tan W (2018) Bioapplications of cell-SELEX-generated aptamers in cancer diagnostics, therapeutics, theranostics and biomarker discovery: a comprehensive review. Cancers 10(2):47
Ma H, Liu J, Ali MM, Mahmood MAI, Labanieh L, Lu M, Iqbal SM, Zhang Q, Zhao W, Wan Y (2015) Nucleic acid aptamers in cancer research, diagnosis and therapy. Chem Soc Rev 44(5):1240–1256
Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L (2003) A tenascin-C aptamer identified by tumor cell SELEX: Systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci USA 100(26):15416–15421
Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W (2006) Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci USA 103(32):11838–11843
Mallikaratchy P, Tang Z, Kwame S, Meng L, Shangguan D, Tan W (2007) Aptamer directly evolved from live cells recognizes membrane bound immunoglobin heavy mu chain in Burkitt’s lymphoma cells. Mol Cell Proteomics 6(12):2230–2238
Xiong H, Yan J, Cai S, He Q, Peng D, Liu Z, Liu Y (2019) Cancer protein biomarker discovery based on nucleic acid aptamers. Int J Biol Macromol 132:190–202
Fang X, Tan W (2010) Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res 43(1):48–57
Li S, Xu H, Ding H, Huang Y, Cao X, Yang G, Li J, Xie Z, Meng Y, Li X, Zhao Q, Shen B, Shao N (2009) Identification of an aptamer targeting hnRNP A1 by tissue slide-based SELEX. J Pathol 218(3):327–336
Mi J, Liu Y, Rabbani ZN, Yang Z, Urban JH, Sullenger BA, Clary BM (2010) In vivo selection of tumor-targeting RNA motifs. Nat Chem Biol 6(1):22–24
Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, Carter J, Dalby AB, Eaton BE, Fitzwater T, Flather D, Forbes A, Foreman T, Fowler C, Gawande B, Goss M, Gunn M, Gupta S, Halladay D, Heil J, Heilig J, Hicke B, Husar G, Janjic N, Jarvis T, Jennings S, Katilius E, Keeney TR, Kim N, Koch TH, Kraemer S, Kroiss L, Le N, Levine D, Lindsey W, Lollo B, Mayfield W, Mehan M, Mehler R, Nelson SK, Nelson M, Nieuwlandt D, Nikrad M, Ochsner U, Ostroff RM, Otis M, Parker T, Pietrasiewicz S, Resnicow DI, Rohloff J, Sanders G, Sattin S, Schneider D, Singer B, Stanton M, Sterkel A, Stewart A, Stratford S, Vaught JD, Vrkljan M, Walker JJ, Watrobka M, Waugh S, Weiss A, Wilcox SK, Wolfson A, Wolk SK, Zhang C, Zichi D (2010) Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 5(12):e15004
Ostroff RM, Bigbee WL, Franklin W, Gold L, Mehan M, Miller YE, Pass HI, Rom WN, Siegfried JM, Stewart A, Walker JJ, Weissfeld JL, Williams S, Zichi D, Brody EN (2010) Unlocking biomarker discovery: large scale application of aptamer proteomic technology for early detection of lung cancer. PLoS ONE 5(12):e15003
Vaught JD, Bock C, Carter J, Fitzwater T, Otis M, Schneider D, Rolando J, Waugh S, Wilcox SK, Eaton BE (2010) Expanding the chemistry of DNA for in vitro selection. J Am Chem Soc 132(12):4141–4151
Baird GS, Nelson SK, Keeney TR, Stewart A, Williams S, Kraemer S, Peskind ER, Montine TJ (2012) Age-dependent changes in the cerebrospinal fluid proteome by slow off-rate modified aptamer array. Am J Pathol 180(2):446–456
Webber J, Stone TC, Katilius E, Smith BC, Gordon B, Mason MD, Tabi Z, Brewis IA, Clayton A (2014) Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscanTM) platform. Mol Cell Proteomics 13(4):1050–1064
Niu G, Chen X (2010) Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets 11(8):1000–1017
Crulhas BP, Karpik AE, Delella FK, Castro GR, Pedrosa VA (2017) Electrochemical aptamer-based biosensor developed to monitor PSA and VEGF released by prostate cancer cells. Anal Bioanal Chem 409(29):6771–6780
Qureshi A, Gurbuz Y, Niazi JH (2015) Capacitive aptamer–antibody based sandwich assay for the detection of VEGF cancer biomarker in serum. Sensors Actuat B Chem 209:645–651
Wang B, Akiba U, Anzai J-i (2017) Recent progress in nanomaterial-based electrochemical biosensors for cancer biomarkers: A review. Molecules 22(7):1048
Shamsipur M, Farzin L, Amouzadeh Tabrizi M, Molaabasi F (2015) Highly sensitive label free electrochemical detection of VGEF165 tumor marker based on “signal off” and “signal on” strategies using an anti-VEGF165 aptamer immobilized BSA-gold nanoclusters/ionic liquid/glassy carbon electrode. Biosens Bioelectron 74:369–375
Amouzadeh Tabrizi M, Shamsipur M, Farzin L (2015) A high sensitive electrochemical aptasensor for the determination of VEGF165 in serum of lung cancer patient. Biosens Bioelectron 74:764–769
Ravalli A, Rivas L, De La Escosura-Muñiz A, Pons J, Merkoçi A, Marrazza G (2015) A DNA aptasensor for electrochemical detection of vascular endothelial growth factor. J Nanosci Nanotechno 15(5):3411–3416
Fu X-M, Liu Z-J, Cai S-X, Zhao Y-P, Wu D-Z, Li C-Y, Chen J-H (2016) Electrochemical aptasensor for the detection of vascular endothelial growth factor (VEGF) based on DNA-templated Ag/Pt bimetallic nanoclusters. Chin Chem Lett 27(6):920–926
Amouzadeh Tabrizi M, Shamsipur M, Saber R, Sarkar S (2017) Simultaneous determination of CYC and VEGF165 tumor markers based on immobilization of flavin adenine dinucleotide and thionine as probes on reduced graphene oxide-poly(amidoamine)/gold nanocomposite modified dual working screen-printed electrode. Sensors Actuat B Chem 240:1174–1181
Da H, Liu H, Zheng Y, Yuan R, Chai Y (2018) A highly sensitive VEGF165 photoelectrochemical biosensor fabricated by assembly of aptamer bridged DNA networks. Biosens Bioelectron 101:213–218
Wang Q-L, Cui H-F, Song X, Fan S-F, Chen L-L, Li M-M, Li Z-Y (2018) A label-free and lectin-based sandwich aptasensor for detection of carcinoembryonic antigen. Sensors Actuat B Chem 260:48–54
Gao Y, Song P, Li H, Jia H, Zhang B (2017) Elevated serum CEA levels are associated with the explosive progression of lung adenocarcinoma harboring EGFR mutations. BMC Cancer 17(1):484
Nguyen HH, Park J, Kang S, Kim M (2015) Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 15(5):10481–10510
Guo C, Su F, Song Y, Hu B, Wang M, He L, Peng D, Zhang Z (2017) Aptamer-templated silver nanoclusters embedded in zirconium metal–organic framework for bifunctional electrochemical and SPR aptasensors toward carcinoembryonic antigen. ACS Appl Mater Inter 9(47):41188–41199
Martin V, Sullivan B, Walker K, Hawk H, Sullivan B, Noe L (2006) Surface plasmon resonance investigations of human epidermal growth factor receptor 2. Appl Spectrosc 60(9):994–1003
Eletxigerra U, Martinez-Perdiguero J, Barderas R, Pingarrón JM, Campuzano S, Merino S (2016) Surface plasmon resonance immunosensor for ErbB2 breast cancer biomarker determination in human serum and raw cancer cell lysates. Anal Chim Acta 905:156–162
Uludag Y, Tothill IE (2012) Cancer biomarker detection in serum samples using surface plasmon resonance and quartz crystal microbalance sensors with nanoparticle signal amplification. Anal Chem 84(14):5898–5904
Qian H, Huang Y, Duan X, Wei X, Fan Y, Gan D, Yue S, Cheng W, Chen T (2019) Fiber optic surface plasmon resonance biosensor for detection of PDGF-BB in serum based on self-assembled aptamer and antifouling peptide monolayer. Biosens Bioelectron 140:111350
Yuan J, Oliver R, Li J, Lee J, Aguilar M, Wu Y (2007) Sensitivity enhancement of SPR assay of progesterone based on mixed self-assembled monolayers using nanogold particles. Biosens Bioelectron 23(1):144–148
Chang C-C, Chiu N-F, Lin DS, Chu-Su Y, Liang Y-H, Lin C-W (2010) High-sensitivity detection of carbohydrate antigen 15-3 using a gold/zinc oxide thin film surface plasmon resonance-based biosensor. Anal Chem 82(4):1207–1212
Yi B, Williams PJ, Niewolna M, Wang Y, Yoneda T (2002) Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res 62(3):917–923
Yang H, Gijs MAM (2018) Micro-optics for microfluidic analytical applications. Chem Soc Rev 47(4):1391–1458
Vance SA, Sandros MG (2014) Zeptomole detection of C-reactive protein in serum by a nanoparticle amplified surface plasmon resonance imaging aptasensor. Sci Rep 4:5129
Ye S, Mao Y, Guo Y, Zhang S (2014) Enzyme-based signal amplification of surface-enhanced Raman scattering in cancer-biomarker detection. TrAC Trends Anal Chem 55:43–54
Bhamidipati M, Cho H-Y, Lee K-B, Fabris L (2018) SERS-based quantification of biomarker expression at the single cell level enabled by gold nanostars and truncated aptamers. Bioconjugate Chem 29(9):2970–2981
Danckwardt S, Hentze MW, Kulozik AE (2013) Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond. J Mol Med (Berl) 91(11):1257–1271
Li JJ, Fang X, Tan W (2002) Molecular aptamer beacons for real-time protein recognition. Biochem Biophys Res Commun 292(1):31–40
Nutiu R, Li Y (2003) Structure-switching signaling aptamers. J Am Chem Soc 125(16):4771–4778
Fredriksson L, Li H, Eriksson U (2004) The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 15(4):197–204
Yu J, Ustach C, Kim H-RC (2003) Platelet-derived growth factor signaling and human cancer. J Biochem Mol Biol 36(1):49–59
Wang X, Jiang A, Hou T, Li H, Li F (2015) Enzyme-free and label-free fluorescence aptasensing strategy for highly sensitive detection of protein based on target-triggered hybridization chain reaction amplification. Biosens Bioelectron 70:324–329
Li X, Ding X, Fan J (2015) Nicking endonuclease-assisted signal amplification of a split molecular aptamer beacon for biomolecule detection using graphene oxide as a sensing platform. Analyst 140(23):7918–7925
Zheng C, Zheng A-X, Liu B, Zhang X-L, He Y, Li J, Yang H-H, Chen G (2014) One-pot synthesized DNA-templated Ag/Pt bimetallic nanoclusters as peroxidase mimics for colorimetric detection of thrombin. Chem Commun 50(86):13103–13106
Xu H, Wu D, Li C-Q, Lu Z, Liao X-Y, Huang J, Wu Z-S (2017) Label-free colorimetric detection of cancer related gene based on two-step amplification of molecular machine. Biosens Bioelectron 90:314–320
Alix-Panabières C, Pantel K (2014) Challenges in circulating tumour cell research. Nat Rev Cancer 14(9):623–631
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559–1564
Shen Z, Wu A, Chen X (2017) Current detection technologies for circulating tumor cells. Chem Soc Rev 46(8):2038–2056
Green BJ, Saberi Safaei T, Mepham A, Labib M, Mohamadi RM, Kelley SO (2016) Beyond the capture of circulating tumor cells: next-generation devices and materials. Angew Chem Int Ed Engl 55(4):1252–1265
Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JWM, Gratama J-W, Sleijfer S, Foekens JA (2009) Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer I 101(1):61–66
Zhao W, Cui CH, Bose S, Guo D, Shen C, Wong WP, Halvorsen K, Farokhzad OC, Teo GSL, Phillips JA (2012) Bioinspired multivalent DNA network for capture and release of cells. Proc Natl Acad Sci USA 109(48):19626–19631
Shen Q, Xu L, Zhao L, Wu D, Fan Y, Zhou Y, OuYang WH, Xu X, Zhang Z, Song M (2013) Specific capture and release of circulating tumor cells using aptamer-modified nanosubstrates. Adv Mater 25(16):2368–2373
Wan Y, Liu Y, Allen PB, Asghar W, Mahmood MAI, Tan J, Duhon H, Kim Y-t, Ellington AD, Iqbal SM (2012) Capture, isolation and release of cancer cells with aptamer-functionalized glass bead array. Lab Chip 12(22):4693–4701
Hasanzadeh M, Shadjou N, de la Guardia M (2015) Recent advances in nanostructures and nanocrystals as signal-amplification elements in electrochemical cytosensing. TrAC Trends Anal Chem 72:123–140
Li S, Liu Y, Ma Q (2019) Nanoparticle-based electrochemiluminescence cytosensors for single cell level detection. TrAC Trends Anal Chem 110:277–292
Lorenzo-Gómez R, Miranda-Castro R, de-los-Santos-Álvarez N, Lobo-Castañón MJ (2019) Electrochemical aptamer-based assays coupled to isothermal nucleic acid amplification techniques: New tools for cancer diagnosis. Curr Opin Electrochem 14:32-43
Du Y, Dong S (2017) Nucleic acid biosensors: recent advances and perspectives. Anal Chem 89(1):189–215
Zhao Y, Xu D, Tan W (2017) Aptamer-functionalized nano/micro-materials for clinical diagnosis: isolation, release and bioanalysis of circulating tumor cells. Integr Biol-UK 9(3):188–205
Pan C, Guo M, Nie Z, Xiao X, Yao S (2009) Aptamer-based electrochemical sensor for label-free recognition and detection of cancer cells. Electroanalysis 21(11):1321–1326
Feng L, Chen Y, Ren J, Qu X (2011) A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials 32(11):2930–2937
Zhang H, Li B, Sun Z, Zhou H, Zhang S (2017) Integration of intracellular telomerase monitoring by electrochemiluminescence technology and targeted cancer therapy by reactive oxygen species. Chem Sci 8(12):8025–8029
Liu S, Zhao S, Tu W, Wang X, Wang X, Bao J, Wang Y, Han M, Dai Z (2018) A “signal on” photoelectrochemical biosensor based on bismuth@ N, O-codoped-carbon core-shell nanohybrids for ultrasensitive detection of telomerase in HeLa cells. Chem Eur J 24(15):3677–3682
Khoshfetrat SM, Mehrgardi MA (2017) Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticles on a nitrogen-doped graphene modified electrode. Bioelectrochemistry 114:24–32
Cao J, Zhao X-P, Younis MR, Li Z-Q, Xia X-H, Wang C (2017) Ultrasensitive capture, detection, and release of circulating tumor cells using a nanochannel–ion channel hybrid coupled with electrochemical detection technique. Anal Chem 89(20):10957–10964
Sun D, Lu J, Zhang L, Chen Z (2019) Aptamer-based electrochemical cytosensors for tumor cell detection in cancer diagnosis: A review. Anal Chim Acta 1082:1–17
Li J, Lin X, Zhang Z, Tu W, Dai Z (2019) Red light-driven photoelectrochemical biosensing for ultrasensitive and scatheless assay of tumor cells based on hypotoxic AgInS2 nanoparticles. Biosens Bioelectron 126:332–338
Amouzadeh Tabrizi M, Shamsipur M, Saber R, Sarkar S (2017) Flow injection amperometric sandwich-type aptasensor for the determination of human leukemic lymphoblast cancer cells using MWCNTs-Pdnano/PTCA/aptamer as labeled aptamer for the signal amplification. Anal Chim Acta 985:61–68
Yi Z, Li X-Y, Gao Q, Tang L-J, Chu X (2013) Aptamer-aided target capturing with biocatalytic metal deposition: an electrochemical platform for sensitive detection of cancer cells. Analyst 138(7):2032–2037
Wang X, Ju J, Li J, Li J, Qian Q, Mao C, Shen J (2014) Preparation of electrochemical cytosensor for sensitive detection of HeLa cells based on self-assembled monolayer. Electrochim Acta 123:511–517
Zhu X, Yang J, Liu M, Wu Y, Shen Z, Li G (2013) Sensitive detection of human breast cancer cells based on aptamer–cell–aptamer sandwich architecture. Anal Chim Acta 764:59–63
Wang Q, Wei H, Zhang Z, Wang E, Dong S (2018) Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal Chem 105:218–224
Amouzadeh Tabrizi M, Shamsipur M, Saber R, Sarkar S, Sherkatkhameneh N (2017) Flow injection amperometric sandwich-type electrochemical aptasensor for the determination of adenocarcinoma gastric cancer cell using aptamer-Au@Ag nanoparticles as labeled aptamer. Electrochim Acta 246:1147–1154
Zheng T, Tan T, Zhang Q, Fu J-J, Wu J-J, Zhang K, Zhu J-J, Wang H (2013) Multiplex acute leukemia cytosensing using multifunctional hybrid electrochemical nanoprobes at a hierarchically nanoarchitectured electrode interface. Nanoscale 5(21):10360–10368
Chen X, He Y, Zhang Y, Liu M, Liu Y, Li J (2014) Ultrasensitive detection of cancer cells and glycan expression profiling based on a multivalent recognition and alkaline phosphatase-responsive electrogenerated chemiluminescence biosensor. Nanoscale 6(19):11196–11203
Sun D, Lu J, Wang X, Zhang Y, Chen Z (2017) Voltammetric aptamer based detection of HepG2 tumor cells by using an indium tin oxide electrode array and multifunctional nanoprobes. Microchim Acta 184(9):3487–3496
Fathi F, Rashidi M-R, Omidi Y (2019) Ultra-sensitive detection by metal nanoparticles-mediated enhanced SPR biosensors. Talanta 192:118–127
Li Y, Zhang Y, Zhao M, Zhou Q, Wang L, Wang H, Wang X, Zhan L (2016) A simple aptamer-functionalized gold nanorods based biosensor for the sensitive detection of MCF-7 breast cancer cells. Chem Commun 52(20):3959–3961
Liu R, Wang Q, Li Q, Yang X, Wang K, Nie W (2017) Surface plasmon resonance biosensor for sensitive detection of microRNA and cancer cell using multiple signal amplification strategy. Biosens Bioelectron 87:433–438
Liang D, Jin Q, Yan N, Feng J, Wang J, Tang X (2018) SERS nanoprobes in biologically Raman silent region for tumor cell imaging and in vivo tumor spectral detection in mice. Adv Biosyst 2(12):1800100
Wang J, Liang D, Feng J, Tang X (2019) Multicolor cocktail for breast cancer multiplex phenotype targeting and diagnosis using bioorthogonal surface-enhanced raman scattering nanoprobes. Anal Chem 91(17):11045–11054
Zou Y, Huang S, Liao Y, Zhu X, Chen Y, Chen L, Liu F, Hu X, Tu H, Zhang L (2018) Isotopic graphene–isolated-Au-nanocrystals with cellular Raman-silent signals for cancer cell pattern recognition. Chem Sci 9(10):2842–2849
Phillips JA, Xu Y, Xia Z, Fan ZH, Tan W (2009) Enrichment of cancer cells using aptamers immobilized on a microfluidic channel. Anal Chem 81(3):1033–1039
Sheng W, Chen T, Kamath R, Xiong X, Tan W, Fan ZH (2012) Aptamer-enabled efficient isolation of cancer cells from whole blood using a microfluidic device. Anal Chem 84(9):4199–4206
Guo S, Huang H, Deng X, Chen Y, Jiang Z, Xie M, Liu S, Huang W, Zhou X (2018) Programmable DNA-responsive microchip for the capture and release of circulating tumor cells by nucleic acid hybridization. Nano Res 11(5):2592–2604
Sun N, Liu M, Wang J, Wang Z, Li X, Jiang B, Pei R (2016) Chitosan nanofibers for specific capture and nondestructive release of CTCs assisted by pCBMA brushes. Small 12(36):5090–5097
Kitov PI, Bundle DR (2003) On the nature of the multivalency effect: a thermodynamic model. J Am Chem Soc 125(52):16271–16284
Zhao L, Tang C, Xu L, Zhang Z, Li X, Hu H, Cheng S, Zhou W, Huang M, Fong A, Liu B, Tseng H-R, Gao H, Liu Y, Fang X (2016) Enhanced and differential capture of circulating tumor cells from lung cancer patients by microfluidic assays using aptamer cocktail. Small 12(8):1072–1081
Sharma S, Zhuang R, Long M, Pavlovic M, Kang Y, Ilyas A, Asghar W (2018) Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol Adv 36(4):1063–1078
Zheng Q, Iqbal SM, Wan Y (2013) Cell detachment: Post-isolation challenges. Biotechnol Adv 31(8):1664–1675
Yu X, Wang B, Zhang N, Yin C, Chen H, Zhang L, Cai B, He Z, Rao L, Liu W, Wang F-B, Guo S-S, Zhao X-Z (2015) Capture and release of cancer cells by combining on-chip purification and off-chip enzymatic treatment. ACS Appl Mater Inter 7(43):24001–24007
Wu L, Xu X, Sharma B, Wang W, Qu X, Zhu L, Zhang H, Song Y, Yang C (2019) Beyond capture: circulating tumor cell release and single-cell analysis. Small Meth 3(5):1800544
Chiu W-J, Ling T-K, Chiang H-P, Lin H-J, Huang C-C (2015) Monitoring cluster ions derived from aptamer-modified gold nanofilms under laser desorption/ionization for the detection of circulating tumor cells. ACS Appl Mater Inter 7(16):8622–8630
Labib M, Green B, Mohamadi RM, Mepham A, Ahmed SU, Mahmoudian L, Chang IH, Sargent EH, Kelley SO (2016) Aptamer and antisense-mediated two-dimensional isolation of specific cancer cell subpopulations. J Am Chem Soc 138(8):2476–2479
Reinholt SJ, Craighead HG (2018) Microfluidic device for aptamer-based cancer cell capture and genetic mutation detection. Anal Chem 90(4):2601–2608
Green BJ, Kermanshah L, Labib M, Ahmed SU, Silva PN, Mahmoudian L, Chang IH, Mohamadi RM, Rocheleau JV, Kelley SO (2017) Isolation of phenotypically distinct cancer cells using nanoparticle-mediated sorting. ACS Appl Mater Inter 9(24):20435–20443
Poudineh M, Labib M, Ahmed S, Nguyen LM, Kermanshah L, Mohamadi RM, Sargent EH, Kelley SO (2017) Profiling functional and biochemical phenotypes of circulating tumor cells using a two-dimensional sorting device. Angew Chem Int Ed 56(1):163–168
Chakravarty R, Goel S, Cai W (2014) Nanobody: the “magic bullet” for molecular imaging? Theranostics 4(4):386–398
Que-Gewirth NS, Sullenger BA (2007) Gene therapy progress and prospects: RNA aptamers. Gene Ther 14(4):283–291
Gomes de Castro MA, Hobartner C, Opazo F (2017) Aptamers provide superior stainings of cellular receptors studied under super-resolution microscopy. PLoS ONE 12(2):e0173050
Melancon MP, Zhou M, Zhang R, Xiong C, Allen P, Wen X, Huang Q, Wallace M, Myers JN, Stafford RJ, Liang D, Ellington AD, Li C (2014) Selective uptake and imaging of aptamer- and antibody-conjugated hollow nanospheres targeted to epidermal growth factor receptors overexpressed in head and neck cancer. ACS Nano 8(5):4530–4538
Yoon S, Rossi JJ (2017) Emerging cancer-specific therapeutic aptamers. Curr Opin Oncol 29(5):366–374
Imam A (1985) Application of immunohistochemical methods in the diagnosis of malignant disease. Cancer Invest 3(4):339–359
Bukari BA, Citartan M, Ch’ng ES, Bilibana MP, Rozhdestvensky T, Tang T-H (2017) Aptahistochemistry in diagnostic pathology: technical scrutiny and feasibility. Histochem Cell Biol 147 (5):545-553
Yoon S, Huang KW, Reebye V, Mintz P, Tien YW, Lai HS, Saetrom P, Reccia I, Swiderski P, Armstrong B, Jozwiak A, Spalding D, Jiao L, Habib N, Rossi JJ (2016) Targeted delivery of C/EBPalpha -saRNA by pancreatic ductal adenocarcinoma-specific RNA aptamers inhibits tumor growth in vivo. Mol Ther 24(6):1106–1116
Duan M, Long Y, Yang C, Wu X, Sun Y, Li J, Hu X, Lin W, Han D, Zhao Y, Liu J, Ye M, Tan W (2016) Selection and characterization of DNA aptamer for metastatic prostate cancer recognition and tissue imaging. Oncotarget 7(24):36436–36446
Pu Y, Liu Z, Lu Y, Yuan P, Liu J, Yu B, Wang G, Yang CJ, Liu H, Tan W (2015) Using DNA aptamer probe for immunostaining of cancer frozen tissues. Anal Chem 87(3):1919–1924
Li W-M, Bing T, Wei J-Y, Chen Z-Z, Shangguan D-H, Fang J (2014) Cell-SELEX-based selection of aptamers that recognize distinct targets on metastatic colorectal cancer cells. Biomaterials 35(25):6998–7007
McDonnell LA, Angel PM, Lou S, Drake RR (2017) Mass spectrometry imaging in cancer research: Future perspectives. Adv Cancer Res 134:283–290
Chen S, Xiong C, Liu H, Wan Q, Hou J, He Q, Badu-Tawiah A, Nie Z (2015) Mass spectrometry imaging reveals the sub-organ distribution of carbon nanomaterials. Nat Nanotechnol 10(2):176–182
Tseng YT, Harroun SG, Wu CW, Mao JY, Chang HT, Huang CC (2017) Satellite-like Gold Nanocomposites for Targeted Mass Spectrometry Imaging of Tumor Tissues. Nanotheranostics 1(2):141–153
Yoon S, Rossi JJ (2018) Targeted Molecular Imaging Using Aptamers in Cancer. Pharmaceuticals 11(3):71
Weissleder R (1999) Molecular Imaging: Exploring the Next Frontier. Radiology 212(3):609–614
Shi H, Tang Z, Kim Y, Nie H, Huang YF, He X, Deng K, Wang K, Tan W (2010) In vivo fluorescence imaging of tumors using molecular aptamers generated by cell-SELEX. Chem Asian J 5(10):2209–2213
Shi H, Cui W, He X, Guo Q, Wang K, Ye X, Tang J (2013) Whole cell-SELEX aptamers for highly specific fluorescence molecular imaging of carcinomas in vivo. PLoS ONE 8(8):e70476
Dassie JP, Hernandez LI, Thomas GS, Long ME, Rockey WM, Howell CA, Chen Y, Hernandez FJ, Liu XY, Wilson ME, Allen LA, Vaena DA, Meyerholz DK, Giangrande PH (2014) Targeted inhibition of prostate cancer metastases with an RNA aptamer to prostate-specific membrane antigen. Mol Ther 22(11):1910–1922
Chen H, Zhao J, Zhang M, Yang H, Ma Y, Gu Y (2015) MUC1 aptamer-based near-infrared fluorescence probes for tumor imaging. Mol Imaging Biol 17(1):38–48
Zhang C, Ji X, Zhang Y, Zhou G, Ke X, Wang H, Tinnefeld P, He Z (2013) One-pot synthesized aptamer-functionalized CdTe:Zn2+ quantum dots for tumor-targeted fluorescence imaging in vitro and in vivo. Anal Chem 85(12):5843–5849
Greenall SA, Donoghue JF, Van Sinderen M, Dubljevic V, Budiman S, Devlin M, Street I, Adams TE, Johns TG (2015) EGFRvIII-mediated transactivation of receptor tyrosine kinases in glioma: mechanism and therapeutic implications. Oncogene 34(41):5277–5287
Tang J, Huang N, Zhang X, Zhou T, Tan Y, Pi J, Pi L, Cheng S, Zheng H, Cheng Y (2017) Aptamer-conjugated PEGylated quantum dots targeting epidermal growth factor receptor variant III for fluorescence imaging of glioma. Int J Nanomedicine 12:3899–3911
Lim EK, Kim B, Choi Y, Ro Y, Cho EJ, Lee JH, Ryu SH, Suh JS, Haam S, Huh YM (2014) Aptamer-conjugated magnetic nanoparticles enable efficient targeted detection of integrin alphavbeta3 via magnetic resonance imaging. J Biomed Mater Res A 102(1):49–59
Zhu H, Zhang L, Liu Y, Zhou Y, Wang K, Xie X, Song L, Wang D, Han C, Chen Q (2016) Aptamer-PEG-modified Fe3O4@Mn as a novel T1- and T2- dual-model MRI contrast agent targeting hypoxia-induced cancer stem cells. Sci Rep 6:39245
Kryza D, Debordeaux F, Azema L, Hassan A, Paurelle O, Schulz J, Savona-Baron C, Charignon E, Bonazza P, Taleb J, Fernandez P, Janier M, Toulme JJ (2016) Ex vivo and in vivo imaging and biodistribution of aptamers targeting the human matrix metalloprotease-9 in melanomas. PLoS ONE 11(2):e0149387
Wu X, Liang H, Tan Y, Yuan C, Li S, Li X, Li G, Shi Y, Zhang X (2014) Cell-SELEX aptamer for highly specific radionuclide molecular imaging of glioblastoma in vivo. PLoS ONE 9(6):e90752
Jacobson O, Weiss ID, Wang L, Wang Z, Yang X, Dewhurst A, Ma Y, Zhu G, Niu G, Kiesewetter DO, Vasdev N, Liang SH, Chen X (2015) 18F-labeled single-stranded DNA aptamer for PET imaging of protein tyrosine kinase-7 expression. J Nucl Med 56(11):1780–1785
Liu K, Song G, Zhang X, Li Q, Zhao Y, Zhou Y, Xiong R, Hu X, Tang Z, Feng G (2017) PTK7 is a novel oncogenic target for esophageal squamous cell carcinoma. World Journal of Surgical Oncology 15(1):105
Wang L, Jacobson O, Avdic D, Rotstein BH, Weiss ID, Collier L, Chen X, Vasdev N, Liang SH (2015) Ortho-stabilized 18F-azido click agents and their application in PET imaging with single-stranded DNA aptamers. Angew Chem Int Ed 54(43):12777–12781
Kim D, Jeong YY, Jon S (2010) A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 4(7):3689–3696
Li CH, Kuo TR, Su HJ, Lai WY, Yang PC, Chen JS, Wang DY, Wu YC, Chen CC (2015) Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci Rep 5:15675
Wu M, Wang Y, Wang Y, Zhang M, Luo Y, Tang J, Wang Z, Wang D, Hao L, Wang Z (2017) Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int J Nanomedicine 12:5313–5330
Zhang J, Smaga LP, Satyavolu NSR, Chan J, Lu Y (2017) DNA Aptamer-Based Activatable Probes for Photoacoustic Imaging in Living Mice. J Am Chem Soc 139(48):17225–17228
Kang WJ, Lee J, Lee YS, Cho S, Ali BA, Al-Khedhairy AA, Heo H, Kim S (2015) Multimodal imaging probe for targeting cancer cells using uMUC-1 aptamer. Colloids Surf B Biointerfaces 136:134–140
Petersen KE, Manangon E, Hood JL, Wickline SA, Fernandez DP, Johnson WP, Gale BK (2014) A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem 406(30):7855–7866
Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W (2015) Exosomes in cancer: small particle, big player. J Hematol Oncol 8(1):83
Jalalian SH, Ramezani M, Jalalian SA, Abnous K, Taghdisi SM (2019) Exosomes, new biomarkers in early cancer detection. Anal Biochem 571:1–13
Cheng N, Du D, Wang X, Liu D, Xu W, Luo Y, Lin Y (2019) Recent advances in biosensors for detecting cancer-derived exosomes. Trends Biotechnol 37(11):1236–1254
Yu X, He L, Pentok M, Yang H, Yang Y, Li Z, He N, Deng Y, Li S, Liu T (2019) An aptamer-based new method for competitive fluorescence detection of exosomes. Nanoscale 11(33):15589–15595
Zhang Z, Tang C, Zhao L, Xu L, Zhou W, Dong Z, Yang Y, Xie Q, Fang X (2019) Aptamer-based fluorescence polarization assay for separation-free exosome quantification. Nanoscale 11(20):10106–10113
Jin D, Yang F, Zhang Y, Liu L, Zhou Y, Wang F, Zhang G-J (2018) ExoAPP: exosome-oriented, aptamer nanoprobe-enabled surface proteins profiling and detection. Anal Chem 90(24):14402–14411
Zhang Q, Wang F, Zhang H, Zhang Y, Liu M, Liu Y (2018) Universal Ti3C2 MXenes based self-standard ratiometric fluorescence resonance energy transfer platform for highly sensitive detection of exosomes. Anal Chem 90(21):12737–12744
Wang H, Chen H, Huang Z, Li T, Deng A, Kong J (2018) DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta 184:219–226
Jiang Y, Shi M, Liu Y, Wan S, Cui C, Zhang L, Tan W (2017) Aptamer/AuNP Biosensor for Colorimetric Profiling of Exosomal Proteins. Angew Chem Int Ed 56(39):11916–11920
Xia Y, Liu M, Wang L, Yan A, He W, Chen M, Lan J, Xu J, Guan L, Chen J (2017) A visible and colorimetric aptasensor based on DNA-capped single-walled carbon nanotubes for detection of exosomes. Biosens Bioelectron 92:8–15
Zhou Q, Rahimian A, Son K, Shin D-S, Patel T, Revzin A (2016) Development of an aptasensor for electrochemical detection of exosomes. Methods 97:88–93
Wang S, Zhang L, Wan S, Cansiz S, Cui C, Liu Y, Cai R, Hong C, Teng IT, Shi M, Wu Y, Dong Y, Tan W (2017) Aptasensor with Expanded Nucleotide Using DNA Nanotetrahedra for Electrochemical Detection of Cancerous Exosomes. ACS Nano 11(4):3943–3949
Yang F, Zuo X, Fan C, Zhang X-E (2018) Biomacromolecular nanostructures-based interfacial engineering: from precise assembly to precision biosensing. Natl Sci Rev 5(5):740–755
Wang Z, Zong S, Wang Y, Li N, Li L, Lu J, Wang Z, Chen B, Cui Y (2018) Screening and multiple detection of cancer exosomes using an SERS-based method. Nanoscale 10(19):9053–9062
Cox JC, Hayhurst A, Hesselberth J, Bayer TS, Georgiou G, Ellington AD (2002) Automated selection of aptamers against protein targets translated in vitro: from gene to aptamer. Nucleic Acids Res 30(20):e108
Kim N, Gan HH, Schlick T (2007) A computational proposal for designing structured RNA pools for in vitro selection of RNAs. RNA 13(4):478–492
Wang Q-L, Cui H-F, Du J-F, Lv Q-Y, Song X (2019) In silico post-SELEX screening and experimental characterizations for acquisition of high affinity DNA aptamers against carcinoembryonic antigen. RSC Adv 9(11):6328–6334
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lv, Q., Cui, H., Song, X. (2021). Aptamers for the Diagnosis of Malign Tumors. In: Dong, Y. (eds) Aptamers for Medical Applications. Springer, Singapore. https://doi.org/10.1007/978-981-33-4838-7_9
Download citation
DOI: https://doi.org/10.1007/978-981-33-4838-7_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-4837-0
Online ISBN: 978-981-33-4838-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)