Abstract
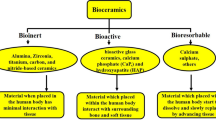
This chapter has a two-fold focus: the first section focuses on the bionic application, and the second section focuses on the tissue scaffold application. Bionics: Alumina is an essential component of the hermetic encapsulation and electrical feedthrough system used in implantable bionics. This has been the case since 1970 when the transition was made from the rudimentary epoxy encapsulation systems used in the developmental era of implantable bionics, to the alumina/titanium hermetic electrical feedthrough system used for all implantable bionics since then. Implantable bionics is a global industry currently worth $25 billion per annum and growing rapidly. Thus, implantable bionics is one of the most important commercial applications for alumina advanced ceramics in the world today. The alumina/titanium hermetic feedthrough system for implantable bionics was invented in 1970 by Author Cowdery and has now become the standard hermetic encapsulation for all bionic implants in the global $25 Billion market. This chapter outlines the science and engineering underlying this feedthrough technology and explores its application in 3 key bionic implants (of the 14 or so types of bionic implants on the market): the pacemaker (Cowdery), bionic ear (Cowdery), and bionic eye (Ruys). Tissue Scaffolds: Alumina is a very strong ceramic, far superior to calcium phosphates, and has a high strength even at porosities of over 90%. It can be rendered bioactive by surface-doping with bioactivity-enhancing ions, such as calcium, phosphorous, magnesium and silicon. Authors Soh and Ruys pioneered a novel doped-porous-alumina tissue scaffold technology with an extremely high porosity of 94.4%, a high compressive strength of 384 MPa, with an average pore size of 300 microns, more than large enough for bone ingrowth. This chapter outlines the science and engineering underlying these developments.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
There is some controversy as to who actually invented the electrical feedthrough concept for the electric light globe, whether it was James Bowman Lindsay in 1835, Marcellin Jobard in 1838, Warren de la Rue in 1840, or Fredrick de Moleyns who patented the first hermetic light globe in 1841.
- 2.
Paralysed woman moves robot with her mind—by Nature Video. https://www.youtube.com/watch?v=ogBX18maUiM&t=0s.
- 3.
The concept and technology of foamed alumina was originally conceived of by Dr Padmaja Parakala, and successfully executed by author Edwin Soh.
References
Aquilina O (2006) A brief history of cardiac pacing. Images Paediatr Cardiol 8:17–81
Wickham GG, Cowdery DJ (1971) An hermetically sealed implantable cardiac pacemaker. In: Proceedings of the 9th international conference on medical and biological engineering, Melbourne
Bondarew V, Seligman P (2012) The Cochlear story (bright ideas series). CSIRO Publishing, Australia
Ruys AJ (2019) Alumina ceramics. Biomedical and industrial applications. Elsevier, Oxford
Jacobi W (1952) Halblieterverstarker. German Patent DE 833366, Filed 1949
Fiandra O (1988) The first pacemaker implant in America. Pacing Clin Electrophysiol 11:1234–1238
Davies JG, Siddons H (1965) Experience with implanted pacemakers: technical considerations. Thorax 20:128–134
Edison TA (1880) Electric lamp. US Patent 223898, Filed 1879
De Forest L (1906) Oscillation-responsive device. US Patent 824637, Filed 1906
De Forest L (1906) Oscillation-responsive device. US Patent 836070, Filed 1906
Scott H (1936) Glass Metal Seal. US Patent 2062335, Filed 1929
Elan Technology (2017) Glass recommendations for Kovar. https://www.elantechnology.com/support/glass-recommendations/kovar-alloy/. Accessed 08 Apr 2021
US Department of Defense (2006) Test method standard—microcircuits, MIL-STD-883G
Vatter H (1937) A method for producing vacuum-tight electric vessels after soldering. German Patent 645871, Filed 1935
Pulfrich H (1939) Ceramic to metal seals. US Patent 2163407, Priority 1936
Nolte HJ (1954) Method of metallizing a ceramic member. US Patent 2,667,427
Burnside DG (1954) Ceramic seals of the tungsten-iron type. RCA Rev 15:46–61
US Department of Defense (2006) Test method standard—test methods for semiconductor devices. MIL-STD-750E
Howl DA, Mann CA (1965) The back-pressurising technique of leak-testing. Vacuum 15:347–352
Cowdery DJ (1969) The pulsed TIG welding process Part 1. Equipment design. Welding Inst Res Bull 10:201–203
Cowdery DJ (1969) Pulsed arc MIG welding with variable pulse width, Report No.672. British Oxygen Company Research Laboratory, Welding Products Division
Mathematica (2017) Electrical conductivity of the elements, Mathematica Element Data, Wolfram Research. https://reference.wolfram.com/language/ref/ElementData.html. Accessed 08 Apr 2021
Zion Market Research (2016) Cardiac pacemaker market by product (implantable cardiac pacemaker and external cardiac pacemaker) by technology (biventricular, single chambered, and dual chambered): global industry perspective, comprehensive analysis and forecast, 2015–2021. https://www.zionmarketresearch.com/report/cardiac-pacemaker-market. Accessed 08 Apr 2021
Elssner G, Pabst R, Puhr-Westerheide J (1974) Schichtverbundkombinationen aus hochschmelzenden metallen und oxiden. Zeitschrift fuer Werkstofft 5:61–69
Sirota NN, Zhabko TE (1981) X-ray study of the anisotropy of thermal properties in titanium. Phys Stat Sol 63:K211–K215
O’Brien B (2015) Niobium biomaterials. In: Niinomi M, Narushima T, Nakai M (eds) Advances in metallic biomaterials. Springer series in biomaterials science and engineering, vol 3. Springer, Heidelberg, pp 245–272
De Bruin HJ, Warble CE (1977) Chemical bonding of metals to ceramic materials. US Patent 4050956, Filed 1975
McAlister AJ, Kahan DJ (1986) The Al−Pt (Aluminum-Platinum) system. Bull All Phase Diagram 7:47–51
Wriedt HA (1985) The Al-O (Aluminum-Oxygen) system. Bull All Phase Diagrams 6:548–553
Allen RV, Borbidge WE (1983) Solid state metal-ceramic bonding of platinum to alumina. J Mater Sci 18:2835–2843
The Engineering Toolbox (2017) Coefficients of linear thermal expansion. http://www.engineeringtoolbox.com/linear-expansion-coefficients-d_95.html. Accessed 08 Apr 2021
Guenther T, Kong C, Lu H et al (2014) Pt-Al2O3 interfaces in cofired ceramics for use in miniaturized neuroprosthetic implants. J Biomed Mater Res B Appl Biomater 102:500–507
Green RA, Guenther T, Jeschke C et al (2013) Integrated electrode and high density feedthrough system for chip-scale implantable devices. Biomaterials 34:6109–6118
Schuettler M, Ordonez JS, Silva Santisteban T et al (2010) Fabrication and test of a hermetic miniature implant package with 360 electrical feedthroughs. Annu Int Conf IEEE Eng Med Biol Soc 2010:1585–1588
Haas LF (2003) Hans Berger (1873–1941), Richard Caton (1842–1926), and electroencephalography. J Neurol Neurosurg Psychiatry 74:9. https://doi.org/10.1136/jnnp.74.1.9
Donoghue J, Hatsopoulos N, Martel S et al (2007) Microstructured arrays for cortex interaction and related methods of manufacture and use. US Patent 7212851
Hochberg LR, Bacher D, Jarosiewicz B et al (2012) Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485:372–375
Leuthardt EC, Schalk G, Moran DW et al (2006) Brain computer interface. US Patent US7120486B2, Filed 2003
Oxley TJ, Opie NL, John SE et al (2016) Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity. Nat Biotechnol 34:320–327
Soh E, Kolos E, Ruys AJ (2015) Cellular response to doping of high porosity foamed alumina with Ca, P, Mg, and Si. Materials 8:1074–1088
Soh E, Kolos E, Ruys AJ (2015) Foamed high porosity alumina for use as a bone tissue scaffold. Ceram Int 41:1031–1047
Soh E, Ruys AJ (2009) Characterisation of foamed porous alumina tissue scaffolds. J Biomim Biomater Tissue Eng 4:21–26
Soh KH, Ruys AJ, Parakala P (2005) Foamed alumina bioceramics for use as tissue scaffolds. In: International Federation for Medical and Biological Engineering (IFMBE) proceedings ICBMEC 2005. The 12th international conference on biomedical engineering, vol 12. Singapore
Pabbruwe MB, Standard OC, Sorrell CC et al (2004) Bone formation within alumina tubes: effect of calcium, manganese, and chromium dopants. Biomaterials 25:4901–4910
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ruys, A.J., Cowdery, D.J., Soh, E.K.L. (2022). Alumina: Implantable Bionics and Tissue Scaffolds. In: Choi, A.H., Ben-Nissan, B. (eds) Innovative Bioceramics in Translational Medicine I. Springer Series in Biomaterials Science and Engineering, vol 17. Springer, Singapore. https://doi.org/10.1007/978-981-16-7435-8_10
Download citation
DOI: https://doi.org/10.1007/978-981-16-7435-8_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7434-1
Online ISBN: 978-981-16-7435-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)